The use of high-protein (HP) diets is a controversial topic. Resistance trainers receive confounding messages about the safety of purposely seeking ample dietary protein in their quest for enhanced protein synthesis, improved performance or maintaining health(Reference Lowery and Devia1). The current dietary reference intake for the general population is 0·8 g/kg body weight per d(Reference Otten and Meyers2). Individuals engaged in regular exercise training require more dietary protein than sedentary individuals(Reference Campbell, Kreider and Ziegenfuss3). Protein intake necessary to support N balance in strength athletes ranges from 1·2 to 1·7 g/kg body weight per d(Reference Campbell, Kreider and Ziegenfuss3–Reference Rodriguez, Di Marco and Langley5). The International Society of Sports Nutrition position stand regarding protein and exercise(Reference Campbell, Kreider and Ziegenfuss3) concluded that the concerns regarding the potential unhealthiness of protein intake within the range of 1·4–2·0 g/kg body weight per d are unfounded in healthy, exercising individuals, and were largely based on data from non-athletes(Reference Lowery and Devia1). However, some other studies have reiterated the tendency towards HP diet consumption by athletes and the potential health hazards posed by such diets(Reference Innocencio da Silva Gomes, Goncalves Ribeiro and de Abreu Soares6).
HP diets appear to reduce energy intake, body-weight gain and fat deposition, and improve the plasma lipid profile(Reference Lacroix, Gaudichon and Martin7–Reference Belobrajdic, McIntosh and Owens11). Whey protein supplements, i.e. above 80 % protein concentrates or above 90 % protein isolates, have become popular among athletes and people interested in gaining muscle mass(Reference Cribb12).
Excessive protein consumption might affect renal health(Reference Jia, Hwang and House13, Reference Friedman14). Nevertheless, it is uncertain whether there is significant evidence to support this relationship in healthy individuals. In fact, some studies suggest that hyperfiltration, the purported mechanism for renal damage, is a normal adaptative mechanism that takes place in response to several physiological conditions(Reference Martin, Armstrong and Rodriguez15). Recently, Jia et al. (Reference Jia, Hwang and House13) have suggested that long-term intakes of protein at the upper limit of the acceptable macronutrient distribution range from whole protein sources may compromise renal health. On the other hand, Frank et al. (Reference Frank, Graf and Amann-Gassner16) detected a significant increment in blood urea N, serum uric acid, glucagon, natriuresis, urinary albumin and urea excretion among healthy young men who consumed HP diets, and concluded that more attention should be paid to the potential adverse renal effects of such diets(Reference Frank, Graf and Amann-Gassner16). Moreover, relative excess of animal protein ingestion and strenuous physical exercise induce intracellular acidosis(Reference Pak17) that stimulates hypocitraturia, which is often accompanied by hypercalciuria(Reference Pak17). Hypercalciuria and hypocitraturia contribute to the formation of Ca-containing kidney stones, mainly by increasing urinary saturation of Ca salts(Reference Pak17, Reference Amanzadeh, Gitomer and Zerwekh18).
Excessive protein consumption may also affect bone health(Reference Barzel and Massey19, Reference Lowe20) due to its acidogenic effect that may result in bone resorption(Reference Weiss, Gorn and Dux21). However, such acidogenic effect may be modified by other nutrients(Reference Massey22). While it is possible that a prolonged HP intake may lead to increased bone resorption and ultimate bone loss, the current evidence on that issue is limited and conflicting. Some studies have reported a negative effect on bone health in both healthy rats(Reference Weiss, Gorn and Dux21, Reference Talbott, Cifuentes and Dunn23) and human subjects(Reference Sellmeyer, Stone and Sebastian24, Reference Kerstetter, Mitnick and Gundberg25). Conversely, other HP studies in healthy rodents(Reference Lacroix, Gaudichon and Martin7, Reference Amanzadeh, Gitomer and Zerwekh18, Reference Mardon, Habauzit and Trzeciakiewicz26) and human subjects of different ages(Reference Alexy, Remer and Manz27, Reference Promislow, Goodman-Gruen and Slymen28) did not show any relationship. In fact, it was reported that a low protein intake could have a negative effect on bone mineral density(Reference Kerstetter, O'Brien and Insogna29).
Resistance training can enhance absolute muscular strength, hypertrophy and muscular power(Reference de Salles, Simao and Miranda30), as well as reduce body fat, lipids and the consequent risk of CVD(Reference Wolfe31–Reference Houston, Fazio and Chilton33). Furthermore, the benefits of exercise on bone health are highly contrasted by numerous studies(Reference Borer34). However, in recent years, muscle dysmorphia has become an emerging condition that primarily affects male bodybuilders. Such individuals obsess about being inadequately muscular. Compulsions include spending hours in the gym, squandering excessive amounts of money on ineffectual sports supplements, abnormal eating patterns or even substance abuse(Reference Mosley35, Reference Knoesen, Thai Vo and Castle36).
Given the complexity of carrying out long-term interventional studies in human subjects and some ethical issues, animal experimental models are often used to study specific research questions. Bone is a complex tissue that changes slowly. As such, it is difficult to design and conduct well-controlled nutrition studies in human subjects to quantify the effect of one nutrient on bone(Reference Kerstetter, O'Brien and Insogna29). The extrapolation of rodent studies to human subjects is widely found in the literature due to similar patterns of bone and renal structure and metabolism in both species(Reference Pak17, Reference Amanzadeh, Gitomer and Zerwekh18, Reference Weiss, Gorn and Dux21, Reference Talbott, Cifuentes and Dunn23, Reference Pye, Wakefield and Aukema37). Furthermore, the use of rodent experimental models is especially useful in bone metabolism, mainly because years, not weeks, are required to assess bone density change in human subjects(Reference Borer34). The present study aimed: (1) to examine the effect of high-whey-protein consumption on body composition, lipid profile, and renal and bone parameters; (2) to examine the effect of resistance training on such outcomes and its potential interactions with a HP diet.
Experimental methods
Animals and experimental design
A total of ninety-six young albino male Wistar rats were allocated into four groups (n 24): normal-protein (NP)-sedentary group, NP-resistance training group, HP-sedentary group and HP-resistance training group. We splitted each group into three lots (n 8) that were killed 1, 2 and 3 months after the start of the experiment, thus resulting in twelve study groups.
The animals, with an initial body weight of 150 (sd 8) g, were housed from day 0 of the experiment in individual stainless steel metabolism cages designed for the separate collection of faeces and urine. The cages were located in a well-ventilated thermostatically controlled room (21 ± 2°C), with a relative humidity ranging from 40 to 60 %. A reverse 12 h light–12 h dark cycle (08.00–20.00 hours) was implemented to allow exercise training during the day. Throughout the experimental period, all rats had free access to double-distilled water and the animals consumed the two different diets ad libitum. One week before the experimental period, the rats were allowed to adapt to the diet and experimental conditions.
Body weight was measured weekly for all animals at the same time, and the amount of food consumed by each rat was registered daily.
On days 21, 49 and 77, a 12 h urine sample from each animal was collected for biochemical analysis. During these 12 h, located in the dark cycle, water was removed in order to avoid interferences with urine collection. Urine volumes were recorded, and samples were transferred into graduated centrifuge tubes for pH, Ca and citrate analysis.
At the end of the experimental period, the animals were anaesthetised with pentobarbital and killed by cannulation of the abdominal aorta. Blood was collected (with heparin as an anticoagulant) and centrifuged at 3000 rpm for 15 min to separate the plasma that was frozen in liquid N2 and stored at − 80°C. Carcass weight was recorded. Carcass is the weight of the slaughtered animal's cold body after being skinned, bled and eviscerated, and after removal of the head, the tail and the feet. Brown and white adipose tissue was extracted and weighed. Kidneys were extracted, weighed and immediately frozen in liquid N2. Femurs, quadriceps and gastrocnemius were extracted, weighed and stored at − 20°C.
All experiments were undertaken according to the Directional Guides Related to Animal Housing and Care (European Community Council, 1986), and all procedures were approved by the Animal Experimentation Ethics Committee of the University of Granada.
Experimental diets
Formulation of the experimental diets is presented in Table 1. All diets were formulated to meet nutrient requirements of rats following the recommendations of the American Institute of Nutrition (AIN-93M)(Reference Reeves, Nielsen and Fahey38), with slight modifications. We selected a protein level of 45 % for the HP diet groups, following previous studies in which an HP diet was compared with NP diets in rats(Reference Lacroix, Gaudichon and Martin7, Reference Pichon, Potier and Tome8, Reference Belobrajdic, McIntosh and Owens11, Reference Amanzadeh, Gitomer and Zerwekh18), whereas a protein content of 10 % was chosen for the NP diet groups. A commercial whey protein isolate was used as the sole source of protein since this protein source is widely available and used by sportsmen. Before diet preparation, total protein concentration of the commercial whey protein hydrolysate and its distribution among the protein or non-protein fractions were measured. Total N content of the commercial whey protein hydrolysate was 11·8 (sd 0·6) g/100 g DM, of which 11·7 % corresponded to insoluble N, whereas 85·4 % corresponded to soluble protein N and 2·9 % to soluble non-protein N.
Table 1 Composition of the experimental diets
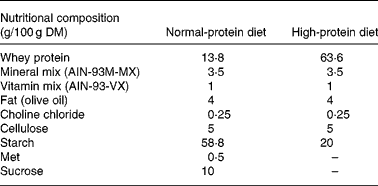
Total protein concentration of the experimental diets was also assayed, with values of 44·3 (sd 2·1) % for the HP diet and 11·7 (sd 0·4) % for the NP diet (these values are adequate for our experimental design).
Chemical analyses
Moisture content was determined by drying to constant weight in an oven at 105 ± 1°C. Total N of the whey protein supplement was determined according to Kjeldahl's method. Crude protein was calculated as N × 6·25. Insoluble N and soluble protein and non-protein N were measured using the methodology described by Periago et al. (1996).
Bone and kidney ash were prepared by calcination at 500°C to a constant weight. Ca content was determined by atomic absorption spectrophotometry using a Perkin Elmer Analyst 300 spectrophotometer (Perkin Elmer, Wellesley, MA, USA). Analytical results were validated by standard references certified reference material (CRM)-189 (wholemeal starch; Community Bureau of Reference, Geel, Belgium), CRM-383 (haricot beans; Community Bureau of Reference) and CRM-709 (pig feed; Community Bureau of Reference). Mean values of five independent values for ash and Ca content were as follows: ash, CRM-383 = 2·48 (sem 0·006) % v. certified value of 2·4 (uncertainty 0·1) %, CRM-709 = 4·29 (sem 0·03) % v. certified value of 4·2 (uncertainty 0·4) %; Ca, CRM-383 = 2·78 (sem 0·02) mg/g v. certified value of 2·9 (uncertainty 0·2) mg/g.
Urinary pH was analysed using a bench pH meter (Crison, Barcelona, Spain). Urinary citrate was measured using a commercial kit. Plasma urea, total cholesterol, TAG and HDL-cholesterol were measured using a HITACHI Roche p800 autoanalyser.
Resistance training
The experimental group was trained following a resistance protocol in a motorised treadmill (Panlab TREADMILLS for five rats, LE 8710R) with weights in a bag tied with a cord to the tail (Fig. 1). The training group exercised on alternate days (3–4 sessions/week). The animals ran at a constant speed of 40 cm/s during the whole experimental period (4, 8 or 12 weeks). Before exercise training, animals were adapted to the treadmill on a daily basis for 1 week, first 3 d without weight and the last 4 d with 20 % of their body weights. The number of sessions performed each week on alternate days, the number of sets per session and the time spent in each set as well as the load carried by the animals are shown in Table 2. From the first week of the experimental period until the completion of the study, the training weights (loads) were progressively increased and individually adjusted one time per week to the percentage of one repetition maximum (1 RM), defined as the maximum load that the rat could carry in the bag. The 1 RM test was conducted as follows: the rat was placed in a flat, horizontal and non-slippery surface with a specific loaded bag that was tied to its tail. The rat was acoustically stimulated and immediately reacted by moving forward. This procedure was repeated several times, increasing the load every time, until the load was so heavy that the rat could not move forward, yet actively stimulated. The load achieved at this point was considered the 1 RM and was weekly measured in all animals to adapt the %1 RM load during the training period. This type of training follows the established principles for human resistance training, involving weights, repetitions and sets to maximise gains in muscle strength(Reference de Salles, Simao and Miranda30). All the training process was designed and controlled by sport scientists in collaboration with experienced researches used to work with rats. We considered that a repetition was finished when the rat stopped running and the next repetition began when the rat started running again, as a consequence of the electric stimulus at the end of the treadmill. The repetitions usually lasted 2–4 s, and the number of repetitions per set ranged between eight and fourteen repetitions (Fig. 1).

Fig. 1 Rats training on the treadmill and details of the resistance training protocol. Number of sets per training session: 10–12. Number of repetitions per set: 8–14. * A repetition is done when the resistance is transported along 20–40 cm or 2–4 s pulling the resistance although no distance is achieved.
Table 2 Details of the resistance training programme
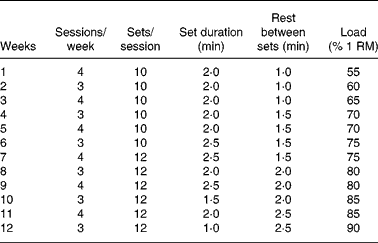
RM, repetition maximum.
Animals in the control groups were managed identically to exercising animals, with the exception of exercise training. In order to avoid a possible confounding effect due to often handling in the training groups, control animals were handled weekly.
Statistical methods
Results are presented as means and standard deviations, unless otherwise indicated. The effects of dietary treatment and resistance training on the outcome variables were analysed by two-way repeated-measures ANOVA; with diet and exercise groups as fixed factors, and pre- and post-intervention values of body composition, lipid profile and renal and bone parameters as dependent variables. All analyses were performed separately for rats killed at 1, 2 and 3 months. All the analyses were performed using the Statistical Package for Social Sciences (SPSS, version 16.0 for Windows; SPSS, Inc., Chicago, IL, USA), and the level of significance was set at P < 0·05.
Results
Food intake
Along the experimental period, food consumption gradually declined in all groups, especially from the second month. Food intake was higher in the NP groups when compared with the HP groups (P < 0·01), and no significant differences were observed between the sedentary and exercise groups.
Body weight, body composition and lipid profile
The effects of NP diet v. HP diet and sedentary status v. resistance training on body weight, body composition and lipid profile are presented in Table 3. Final body weight was lower in the training and NP groups when compared with the sedentary and HP groups, respectively, particularly after 2–3 months of intervention (P < 0·01). There was a significant interaction between diet and training (P < 0·05), with a larger training-derived weight reduction in the NP groups than in the HP groups ( − 11·4 v. − 5·6 % after the second month and − 7·5 v. − 6·0 % after the third month). Carcass weight did not show differences between the sedentary and training groups, while it was heavier in the HP groups compared with the NP groups, particularly after the second and third month of the experimental period (P < 0·01). White fat percentage (related to carcass weight) was lower in the HP groups when compared with the NP groups, especially after the third month (P < 0·05), and in the training groups compared with the sedentary groups, with a larger effect on the second and third month.
Table 3 Effect of normal-protein diet v. high-whey-protein diet and sedentary status v. resistance training on body weight, body composition and metabolic parameters in rats
(Mean values, standard deviations and percentages)

* Percentage of difference between the sedentary and exercise groups was computed as ((exercise − sedentary)/sedentary) × 100.
† Percentage of white fat related to carcass.
Muscle weight (quadriceps and gastrocnemius) was generally higher for the HP groups and especially for the training groups, with a more stable and strong effect after the second month.
Plasma cholesterol levels were lower for the HP groups compared with the NP groups (P < 0·001), and the training groups v. the sedentary groups (P < 0·01 in the first 2 months and P < 0·05 after the third month). There was a significant interaction between diet and training (P < 0·05) on the levels of plasma TAG, with a larger training-derived reduction of plasma TAG in the HP groups when compared with the NP groups ( − 21·1 v. 10·4 % after the second month and − 41·6 v. − 9·0 % after the third month). HDL-cholesterol levels were considerably lower in the HP groups (P < 0·001) when compared with the NP groups, whereas no differences were observed between the exercise and sedentary groups.
Urinary parameters and kidney weight
The effects of the NP diet v. the HP diet and sedentary status v. resistance training on plasma urea and kidney weight are expressed in Table 4. After 3 months of the experimental period, there were significant diet and exercise effects on plasma urea, which was higher in the NP groups v. the HP groups and lower in the exercise groups v. the sedentary groups (P < 0·001). Moreover, a diet × exercise interaction was found, with a higher reduction in urea plasma levels caused by training in the HP groups when compared with the NP groups ( − 37·6 v. − 9·9 %, respectively) (P < 0·01). The training effects on plasma urea were lower in both the HP and the NP experimental groups after the second and third month.
Table 4 Effect of normal-protein diet v. high-whey-protein diet and sedentary status v. resistance training on urea and kidney weight
(Mean values, standard deviations and percentages)

* Percentage of difference between the sedentary and exercise groups was computed as ((exercise − sedentary)/sedentary) × 100.
Kidney weight was higher in the HP groups when compared with the NP groups from the first month of the experimental period (P < 0·001), whereas resistance training caused a significant reduction in the above-mentioned parameter after the third month of the experimental period. At that time, there was a significant diet × exercise interaction due to the more consistent effect of training on the HP group v. the NP group ( − 14·8 v. − 3·3 % reduction, respectively). The same trend was observed when kidney wet mass values were expressed related to carcass weight.
Additionally (data not shown), after the second month of the experimental period, we analysed some urinary parameters, especially those related to metabolic acidity, such as urinary volume, urinary pH, urinary Ca and urinary citrate. Urine volumes were higher in the HP groups at all time points (P < 0·001), without significant differences related to resistance training. Urinary pH was lower in rats that consumed the HP diets compared with those that consumed the NP diets (P < 0·001). Urinary citrate was lower in the HP groups (P < 0·001) when compared with the NP groups, whereas urinary Ca was significantly higher in the former groups (P < 0·01). Regarding exercise effects, both urinary pH and Ca were slightly lower in the resistance training groups, although this effect was not statistically significant.
Bone parameters
The effects of the NP diet v. the HP diet and sedentary status v. resistance training on bone parameters are presented in Table 5. Femur dry weight, total ash and ash per g of DM were generally higher in the HP groups compared with the NP groups (P < 0·05), and a similar effect was observed in the resistance training groups compared with the sedentary groups, although this effect was only significant for femur ash weight (mg/g dry femur) at the third month (P < 0·05). No significant differences in femur Ca content related to diet or training were found.
Table 5 Effect of normal-protein diet v. high-whey-protein diet and sedentary status v. resistance training on bone parameters
(Mean values, standard deviations and percentages)

* Percentage of difference between the sedentary and exercise groups was computed as ((exercise − sedentary)/sedentary) × 100.
Discussion
The main findings of the present study were (1) resistance training caused muscular hypertrophy and significantly reduced total cholesterol and TAG, with a more pronounced effect in the HP group. Resistance training was also effective at enhancing bone mineral content, as measured by femur ash weight (relative to dry femur weight). The effects of training were generally observed at the second and third month, suggesting a mid- to long-term effect. (2) HP diets showed a protective role on the bone mineral content and the lipid profile. (3) Some interactions between diet and training were found, such as the greater effects of resistance training on the lipid profile and kidney weight of rats that consumed the HP diet. (4) Serum and urinary markers showed metabolic acidity derived from high-whey-protein diet consumption; a fact that could explain the increased kidney weight observed in the HP groups. (5) Despite this higher urinary and plasma acidosis in the HP groups, bone was not affected.
Food consumption, body weight, body composition and lipid profile
In a similar way to what has been reported by other authors(Reference Lacroix, Gaudichon and Martin7, Reference Pichon, Potier and Tome8, Reference Belobrajdic, McIntosh and Owens11, Reference Bouthegourd, Roseau and Makarios-Lahham39), food intake and body weight were altered by HP diet consumption. Body weight reduction may be attributed to a combined effect of the lower energy intake exhibited by the HP experimental groups, and the higher energy expenditure required by digestion and metabolism of protein foodstuffs. Alterations in body weight and food intake caused by HP diet consumption were probably on the basis of the lower plasma total- and HDL-cholesterol, and TAG exhibited by HP-fed animals. Furthermore, several human(Reference Cribb, Williams and Stathis9, Reference Hayes and Cribb10) and rodent studies(Reference Pichon, Potier and Tome8, Reference Belobrajdic, McIntosh and Owens11) have demonstrated the ability of whey protein to improve body composition (increasing muscle mass and/or reducing body-weight gain and adiposity index). To note is that in the present study, the HP groups showed higher muscle mass (measured in quadriceps and gastrocnemius). Hypertrophy was also observed, as was expected, on the training groups, especially after 2 months of training.
Lower levels of total cholesterol and TAG could have a protective effect on cardiovascular and kidney disease(Reference Houston, Fazio and Chilton33, Reference Moinuddin and Leehey40, Reference Bianchi, Penno and Romero41). Furthermore, we used whey protein, which appears to be especially indicated to avoid overweight and increased insulin sensitivity(Reference Pichon, Potier and Tome8, Reference Belobrajdic, McIntosh and Owens11). On the other hand, the exercise groups generally presented lower values of cholesterol and TAG, a fact that confirms the highly contrasted effects of resistance training exercise on body composition and lipid profile(Reference Wolfe31–Reference Houston, Fazio and Chilton33). Especially noticeable is the interaction between diet and training on plasma TAG, a finding that points to a potentially more beneficial buffering effect of resistance training on plasma TAG of individuals who consume HP diets and could be at a higher risk of nutritional imbalance and renal alterations.
Renal parameters
Urea, the major end product of protein metabolism in mammals, is the most abundant solute in urine. Plasma urea concentration and glomerular filtration rate increase when normal rats are fed HP diets(Reference Bankir, Bouby and Trinh-Trang-Tan42). More urea needs to be filtered, either because more of it has to be excreted, or because the efficiency of its excretion is reduced. This hyperfiltration might have deleterious consequences in diseased kidneys(Reference Bankir, Bouby and Trinh-Trang-Tan42). In fact, in the recent study of Jia et al. (Reference Jia, Hwang and House13) performed in pigs, after 8 months of the experimental period, renal and glomerular volumes were 60–70 % higher for the HP group. These enlarged kidneys had greater evidence of histological damage, with 55 % more fibrosis and 30 % more glomerulosclerosis. Renal monocyte chemoattractant protein-1 and plasma homocysteine levels were also higher in the HP group. In the present study, kidneys from rats that consumed the HP diet were about 30 % higher. Furthermore, Hammond & Janes(Reference Hammond and Janes43) found a 26–32 % increase in kidney fresh weight of rats after 2 weeks of HP diet consumption which they attributed to the strong effects on blood urea N and totally daily N filtration rate exerted by HP consumption.
On the other hand, in the study of Walrand et al. (Reference Walrand, Short and Bigelow44) performed in human subjects, younger subjects significantly increased their glomerular filtration rate following a HP diet, whereas older subjects did not show the same adaptation and showed a trend towards reduced glomerular filtration rate, thus clearly demonstrating a significant age-related difference in the response to HP intake.
In the present study, resistance exercise decreased kidney weight after 3 months of training, with a higher reduction in the HP experimental groups when compared with the NP experimental groups. A possible explanation could be that resistance training has been reported to reduce inflammation, increase serum albumin and increase glomerular filtration rate(Reference Moinuddin and Leehey40, Reference Poortmans and Ouchinsky45). In the present study, a relatively short-term HP diet consumption (2 months) significantly increased plasma urea, urinary volume and urinary excretion of Ca, but at the same time, it decreased urinary pH and citrate. Since a decrease in urinary pH, hypocitraturia and hypercalcaemia are recognised risk factors for kidney stone formation(Reference Pak17, Reference Amanzadeh, Gitomer and Zerwekh18), and such a tendency was observed in HP-fed rats under our experimental conditions, those animals could also be at risk of nephrolithiasis.
Bone parameters
The effects of HP diet consumption on bone health status appear to be a controversial issue, with authors either reporting a deleterious influence in healthy human subjects(Reference Sellmeyer, Stone and Sebastian24, Reference Kerstetter, Mitnick and Gundberg25) and rats(Reference Weiss, Gorn and Dux21, Reference Talbott, Cifuentes and Dunn22), or describing no adverse effects of HP supplements in rats(Reference Lacroix, Gaudichon and Martin7, Reference Amanzadeh, Gitomer and Zerwekh18, Reference Mardon, Habauzit and Trzeciakiewicz26, Reference Matsuo, Nozaki and Okamura46) and human subjects(Reference Alexy, Remer and Manz27, Reference Promislow, Goodman-Gruen and Slymen28). In this regard, Heaney(Reference Heaney47) have shown that HP diets have adverse effects on health only if dietary Ca and K intakes are not at the recommended levels. On the other hand, a negative relationship between low-protein diet consumption and bone mineral content has been reported(Reference Kerstetter, O'Brien and Insogna29, Reference Hannan, Tucker and Dawson-Hughes48, Reference Heaney49). Under the experimental conditions of the present study, it was interesting to find that the HP diet did not negatively affect bone health parameters such as ash content, but rather had a moderately protective effect on it.
As expected, urinary excretion of Ca was higher in the HP-fed experimental groups. Urinary Ca excretion is strongly related to net renal acid excretion(Reference Massey22, Reference Tylavsky, Spence and Harkness50). The catabolism of dietary protein generates ammonium ion and sulphates from sulphur-containing amino acids. Bone citrate and carbonate are mobilised to neutralise these acids, so urinary Ca increases when dietary protein increases(Reference Massey22, Reference Tylavsky, Spence and Harkness50). Protein is considered to be a net acid-producing substance and thus a net negative risk factor for bone dissolution. However, the acidogenic role of dietary protein remains a matter of debate, as it is well established that other food components have a clear impact on the acid–base balance(Reference Remer51, Reference Remer52).
There is substantial literature supporting the beneficial effects of HP consumption on skeletal metabolism when such protein is consumed together with adequate Ca, K and other minerals, regardless of the source of protein(Reference Massey22, Reference Tylavsky, Spence and Harkness50). Pye et al. (Reference Pye, Wakefield and Aukema37) found that lower body weight, fat mass and higher lean body mass were associated with a high-mixed-protein consumption by rats, but did not find any deleterious effect on bone. The authors concluded that a mixed HP diet containing adequate Ca levels and up to 35 % of energy can be deemed safe for long-term bone health.
Resistance training benefits on bone mineral content have been largely demonstrated(Reference Borer34). In the present study, resistance training was also effective at enhancing bone mineral content, as measured by femur ash weight (relative to dry femur weight). These effects were observed after 3 months of intervention, suggesting a mid- to long-term effect. In the study of Bennell et al. (Reference Bennell, Page and Khan53) performed in rats, the authors did not appreciate differences in the bone of rats developing a similar training protocol. On the other hand, Burr et al. (Reference Burr, Robling and Turner54) reported that short periods of interrupted resistance training, with rest periods between them, were a more effective osteogenic stimulus than a single sustained session. Our training protocol meets effective characteristics mentioned by the authors.
The potential effects of HP diet consumption and strength training on bone health should be considered in relation to new and adjusted protein requirements. As a matter of fact, Elango et al. (Reference Elango, Humayun and Ball55) have shown that dietary reference intake recommendations for mean and population-safe protein intakes of 0·66 and 0·8 g/kg per d, respectively, in adult human subjects are based on a meta-analysis of N balance studies using single linear regression analysis. The authors reanalysed existing N balance studies using two-phase linear regression analysis and obtained mean and safe protein requirements of 0·91 and 0·99 g/kg per d, respectively. The mean and population-safe requirements in adult men were determined to be 0·93 and 1·2 g/kg per d; requirements that are 41 and 50 % higher, respectively, than the current dietary reference intake recommendations(Reference Elango, Humayun and Ball55).
Limitation and strengths
The present study has several limitations that need to be mentioned. First, the study lacked high technology instruments for the measurement of body composition and bone mineral density, such as dual-energy X-ray absorptiometry or the Universal Testing Machine for measuring femoral failure load. Second, the present physiological results obtained in rodents must be confirmed in human subjects. In other words, the effect seen over 2–3 months of a HP diet in rodents cannot be extrapolated directly to the potential effects over decades in human subjects. On the other hand, the present study involved an important number of rats allocated in different groups, so that the main effects of the HP diet, the main effect of resistance training and the interaction between them provide a good opportunity to comprehensively investigate how these lifestyle factors can influence some important health-related outcomes. In addition, some rats were killed after 1 month of intervention, others after the second month and others after the third month. These sequential killings allowed us to study whether diet and/or resistance training effects take place at a shorter or longer term.
In conclusion, high levels of whey protein consumption induced metabolic acidosis in rats, and seemed to negatively affect kidney, but not bone status. In fact, the HP diet showed a moderate positive effect on bone mineral content, as well as on other important health-related outcomes such as plasma lipid profile. The benefits of resistance training were clearly observed on several physiological parameters such as plasma total cholesterol, TAG and bone mineral content in both dietary protein levels studied. However, higher benefits were observed in the lipid profile and renal status of a more potentially compromised health situation such as that of experimental groups fed the HP diets.
Acknowledgements
The authors gratefully acknowledge Encarnación Rebollo and all the members from the Department of Physiology for their collaboration, especially to the persons involved in the fieldwork for their efforts and great enthusiasm. The present study was financially supported by the project 2007/20SVC of the Andalusian Center of Sport Medicine and grants from the Spanish Ministry of Education (EX-2008-0641) and Ministry of Science and Innovation (BES-2009-013442). The authors declare that they have no competing interests. V. A. A. was involved in the conception, planning and designing the study, the acquisition of the data, analysis and interpretation of the data, and drafting the manuscript. E. N. was involved in the acquisition of the data. J. M. P. was involved in organising the research, the acquisition of data, analysis and interpretation of data, and drafting the manuscript. F. B. O. was involved in the analysis and interpretation of the data and drafting the manuscript. J. M. H. was involved in the acquisition of the data. M. L.-J. was involved in the conception, planning and designing the study. P. A. was involved in the conception, planning and designing the study, the acquisition of the data, organising the research and writing the manuscript. All authors read and approved the final manuscript.










