With the increasing prevalence of obesity, sedentary lifestyles and urbanisation, type 2 diabetes mellitus (T2DM) has become a global health issue, affecting 463 million people in 2019, and is predicted to reach 700 million cases in 2045(Reference Williams, Karuranga and Malanda1). T2DM can lead to a series of additional complications, particularly micro- and macro-vascular damage, and negatively affecting multiple vital organs, including the kidneys, liver, eyes and cardiovascular system(Reference Zheng, Ley and Hu2).
Studies have reported that 20–40 % of patients with diabetes suffer from renal dysfunction, characterised by urine albumin excretion or reduced glomerular filtration rate (GFR), and 40 % of them may progress to end-stage renal disease(Reference Aldemir, Turgut and Gokce3–Reference Magee, Hunter and Cardwell6). The exact cause of diabetic renal impairment is complex and is proposed to be contributed to hyperglycaemia, dyslipidaemia, atherosclerotic vascular, obesity, hyperuricaemia, and increased systemic and intra-glomerular pressure(Reference Miranda-Díaz, Pazarín-Villaseñor and Yanowsky-Escatell7,Reference Wolf8) .
Accumulating evidence also indicates that the liver, as an insulin-sensitive tissue and the main regulator of metabolism, is prone to damage by hyperglycaemia, leading to further impaired metabolism and inflammatory reactions(Reference Leclercq, Morais and Schroyen9,Reference Palsamy, Sivakumar and Subramanian10) . Steatosis, elevated liver enzymes, cirrhosis and carcinoma are among several important liver abnormalities in patients with T2DM(Reference Guven, Yavuz and Cam11,Reference Mohamed, Nafizah and Zariyantey12) .
The most well-known strategy to prevent the progression of diabetes-related complications is maintaining glycaemic control(Reference Rask-Madsen and King13). In addition to weight control, lifestyle modifications and medical solutions, there is evidence supporting the effect of gut microbiome in regulating metabolism and energy haemostasis(Reference Heiss and Olofsson14,Reference Xiao and Kang15) . Recently, studies reported alternations of gut microbiota in patients with diabetes(Reference Qin, Li and Cai16–Reference Soyucen, Gulcan and Aktuglu-Zeybek18), and probiotics/synbiotics supplementation was able to exert beneficial effects on lipid profile, glycaemic control, blood pressure and inflammation in these patients(Reference Ardeshirlarijani, Tabatabaei-Malazy and Mohseni19–Reference Yao, Zeng and He25).
The exact mechanism of beneficial effects manifest following probiotic supplementation is not well known. However, its anti-inflammatory properties are very likely contributory. A recent meta-analysis study showed that probiotic therapy significantly decreased C-reactive protein concentration and increased serum levels of glutathione, malondialdehyde and total antioxidant capacity in patients with chronic kidney diseases(Reference Zheng, Guo and Wang22). Moreover, probiotics may improve insulin resistance by increasing liver natural killer T cells and down-regulating TNF-α and NF-κB activity(Reference Ma, Hua and Li26). Probiotics have also shown angiotensin-converting enzyme inhibitor properties, and consequential antihypertensive effects(Reference Qi, Nie and Zhang20,Reference Seppo, Jauhiainen and Poussa27) .
Although there is evidence regarding the beneficial effects of probiotics/synbiotics on the improvement of metabolic control in patients with diabetes(Reference Kocsis, Molnár and Németh24,Reference Yao, Zeng and He25,Reference Bohlouli, Namjoo and Borzoo-Isfahani28–Reference Wang, Zhang and Li30) , so far, no study has systematically examined the effects of probiotics/synbiotics on renal and liver function in these patients. Therefore, we sought to investigate whether probiotic supplementation could improve renal and liver biomarkers, by conducting a systematic and meta-analysis of randomised controlled trials (RCT).
Methods
We performed the present meta-analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and(Reference Higgins, Thomas and Chandler31) adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(Reference Liberati, Altman and Tetzlaff32). This review was registered at the centre of Open Science Framework as https://doi.org/10.17605/OSF.IO/UKXBD.
Search strategy
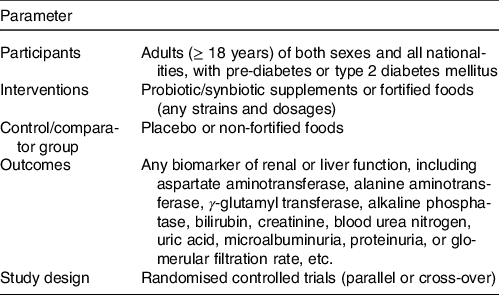
We searched for references indexed in PubMed, Scopus, Web of Science and Cochrane Library, from database inception to 10 February 2021. The terms used in search strategy are provided in online Supplementary Table S1. We did not impose any keywords in terms of interested outcomes and did not apply any restriction for language or publication year. The reference lists of the meta-analyses that examined the effect of probiotic or synbiotic supplementation/fortified foods in T2DM were also searched manually. A specific question was also defined according to the Participants, Interventions, Control, Outcomes and Study design principle (Table 1).
Table 1. Participants, Interventions, Control, Outcomes and Study design criteria for inclusion and exclusion of studies
Selection criteria
The titles/abstracts and full text of retrieved references were screened according to the inclusion and exclusion criteria independently by two authors (SS and FM), and any discrepancies were resolved by discussion with a third author (SA). The inclusion criteria of this article were as follows: the RCT (parallel or crossover) that compared the effects of probiotic/synbiotic supplements or fortified foods (any strains and dosages) with placebo in pre-diabetic or T2DM patients. All included studies needed to report mean and standard deviation of baseline, post or change from baseline for at least one of the following liver enzymes or kidney function indicators, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase, alkaline phosphatase (ALP), bilirubin, creatinine, blood urea nitrogen (BUN), uric acid, microalbuminuria, proteinuria or GFR, or any other renal and liver biomarkers. The exclusion criteria were as follows: (1) trials with less than 1-week period, (2) trials without a placebo-controlled group, (3) duplicated publications from the same population, (4) trials with insufficient information for calculating the mean or standard deviation change in the outcome measure(s), (5) trials including pregnant or lactating women and (6) trials that used probiotic or synbiotic in combination with other treatments and/or the comparator group did not received the same treatment.
Data extraction
The relevant data were extracted by one author and then cross-checked by another (SS, FM), and any discrepancies resolved by discussion with a third author (SA). The following data were extracted: the first author’s name, year of publication, study characteristic (study design, follow-up duration, study location, sample size in the intervention and control groups, the species and dosage of probiotic or synbiotic supplementation and interested outcomes) and participant characteristic (age, sex, health status). The means, along with the respective SD values, of before and after the intervention or change for AST (U/L), ALT (U/L), γ-glutamyl transferase (U/L), ALP (U/L), bilirubin (mg/dl), creatinine (mg/dl), BUN (mg/dl), microalbuminuria (albumin/creatinine ratio), GFR (ml/min per 1·73 m2) and any other liver or renal-related biomarkers also were extracted.
Study quality and quality of evidence
The quality of the selected articles was evaluated using the Cochrane Collaboration’s tool for assessing risk of bias(Reference Higgins, Altman and Gøtzsche33). The quality of evidence assessment was performed with the use of the Grading of Recommendations Assessment, Development and Evaluation approach, which includes five domains: risk of bias, inconsistency of results, imprecision of results, indirectness of evidence and publication bias. The quality of evidence of RCT was initially considered as high and was downgraded by the following limitations: methodological errors(Reference Guyatt, Oxman and Vist34), inconsistency(Reference Guyatt, Oxman and Kunz35), imprecision of estimates(Reference Guyatt, Oxman and Kunz36), indirectness(Reference Guyatt, Oxman and Kunz37) or evidence of publication bias(Reference Guyatt, Oxman and Montori38). All quality evaluation and evidence were performed independently by two reviewers (SS and FM), and disagreements were resolved through discussion with a third author (SA).
Statistical analyses
For each outcome, where at least ≥ 3 RCT reported sufficient data, the net change in mean and its 95 % CI between the intervention and control groups as the effect size is calculated in the meta-analysis. In term of trials that did not provide change values, the mean change was calculated by the minus mean final value from baseline mean value in each arm, and standard deviation of the mean change estimated formula suggested by the Cochrane Handbook of Systematic Review(Reference Higgins, Thomas and Chandler39) where correlation coefficient was imputed (r 0·68 ALP(Reference Abbasi, Ghiasvand and Mirlohi40), r 0·42 AST(Reference Asemi, Aarabi and Hajijafari41–Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43), r 0·48 ALT(Reference Asemi, Aarabi and Hajijafari41), r 0·73 bilirubin(Reference Asemi, Aarabi and Hajijafari41,Reference Asemi, Khorrami-Rad and Alizadeh42) , r 0·82 creatinine(Reference Abbasi, Mirlohi and Daniali44–Reference Soleimani, Zarrati Mojarrad and Bahmani48), r 0·71 BUN(Reference Arani, Emam-Djomeh and Tavakolipour45–Reference Mafi, Namazi and Soleimani47,Reference Soleimani, Motamedzadeh and Mojarrad49) , r 0·77 microalbuminuria(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Ebrahimi, Nasli-Esfahani and Nadjarzade46) , r 0·82 GFR(Reference Abbasi, Mirlohi and Daniali44,Reference Soleimani, Zarrati Mojarrad and Bahmani48) ) from included studies reporting both baseline, final values and changes from baseline for each interested outcome. The random-effects model described by Dersimonian and Laird was used to calculate the overall pooled effect(Reference DerSimonian and Laird50).
Regarding trials that multiple intervention (probiotic or synbiotic) compared with the single control group, the calculated effect size related to probiotic supplementation was included in main analysis to avoid counting the control group twice in the analysis.
Inconsistencies across trials were assessed with the use of the Cochrane’s χ 2 test and the I 2 statistic, where significant heterogeneity was evident as I 2 ≥ 50 %(Reference Deeks, Higgins and Altman51,Reference Higgins, Thompson and Deeks52) . The subgroup analyses were conducted to detect source of heterogeneity if there are adequate trials for each outcome. Sensitivity analysis was conducted to evaluate the impacts of each trial on the meta-analysis results. The presence of publication bias was evaluated by the ‘Begg’s funnel plot’ and Egger’s test whenever if possible (at least ten trials included)(Reference Egger and Smith53,Reference Sterne, Egger and Smith54) . Statistical analyses were conducted using STATA version 14 (STATA Corp.). Two-tailed P values of 0·05 were, a priori, considered as statistically significant.
Results
Study selection and characteristics
The study selection process is detailed in Fig. 1. Our initial systematic search identified 4905 potentially relevant studies, after removing duplicates (n 1348). Following title/abstract review, ninety-eight articles were retained for full-text screening, and then, eighty-three further articles were excluded due to the wrong population (n 4), wrong intervention (n 16), wrong outcome (n 51), wrong comparison (n 2), insufficient data (n 1), repeated reports (n 6) and without full text (n 3). The excluded studies as well as the reasons are shown in online Supplementary Table S2. Finally, fifteen trials were eligible for inclusion in the systematic review and reported following outcomes: ALP (n 4), ALT (n 6), AST (n 6), bilirubin (n 3), BUN (n 5), creatinine (n 6), GFR (n 3), microalbuminuria (n 3), uric acid (n 2), cystatin-C (n 1), albumin (n 1), γ-glutamyl transferase (n 1) and neutrophil gelatinase-associated lipocalin (n 1).

Fig. 1. Study selection process.
The study characteristics are described in Table 2. Except for two studies(Reference Asemi, Aarabi and Hajijafari41,Reference Asemi, Khorrami-Rad and Alizadeh42) , all the included studies were parallel in design. Most of the included studies were carried out in Iran(Reference Abbasi, Ghiasvand and Mirlohi40–Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Asemi, Bahmani and Shakeri55–Reference Miraghajani, Zaghian and Mirlohi57) , and the rest of the studies were performed in Ukraine(Reference Kobyliak, Abenavoli and Mykhalchyshyn58), Sweden(Reference Mobini, Tremaroli and Ståhlman59) and Malaysia(Reference Firouzi, Mohd-Yusof and Majid60). Participants were composed of both male and female in all the included studies and were with T2DM, although patients with both type 1 and type 2 diabetes were eligible for inclusion in two studies(Reference Mafi, Namazi and Soleimani47,Reference Soleimani, Zarrati Mojarrad and Bahmani48) , and one study did not provide information about the type of diabetes(Reference Arani, Emam-Djomeh and Tavakolipour45). Participants in seven studies suffered from nephropathy(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Abbasi, Mirlohi and Daniali44,Reference Arani, Emam-Djomeh and Tavakolipour45,Reference Mafi, Namazi and Soleimani47,Reference Miraghajani, Zaghian and Mirlohi57,Reference Mobini, Tremaroli and Ståhlman59) , dialysis(Reference Soleimani, Zarrati Mojarrad and Bahmani48) and non-alcoholic fatty liver(Reference Kobyliak, Abenavoli and Mykhalchyshyn58). The mean baseline BMI presented an obesity (> 30 kg/m2) condition in six studies(Reference Asemi, Khorrami-Rad and Alizadeh42,Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43,Reference Arani, Emam-Djomeh and Tavakolipour45,Reference Asemi, Zare and Shakeri56,Reference Kobyliak, Abenavoli and Mykhalchyshyn58,Reference Mobini, Tremaroli and Ståhlman59) , and participants in other studies were in overweight category. Participants in five studies were treated with exogenous insulin(Reference Mafi, Namazi and Soleimani47,Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Miraghajani, Zaghian and Mirlohi57–Reference Mobini, Tremaroli and Ståhlman59) , and oral anti-hyperglycaemic drugs were given in rest of the studies.
Table 2. The characteristics of trials that investigated the effect of probiotics/synbiotics supplementation on liver and renal biomarkers in adults with type 2 diabetes and were eligible for inclusion in the meta-analysis
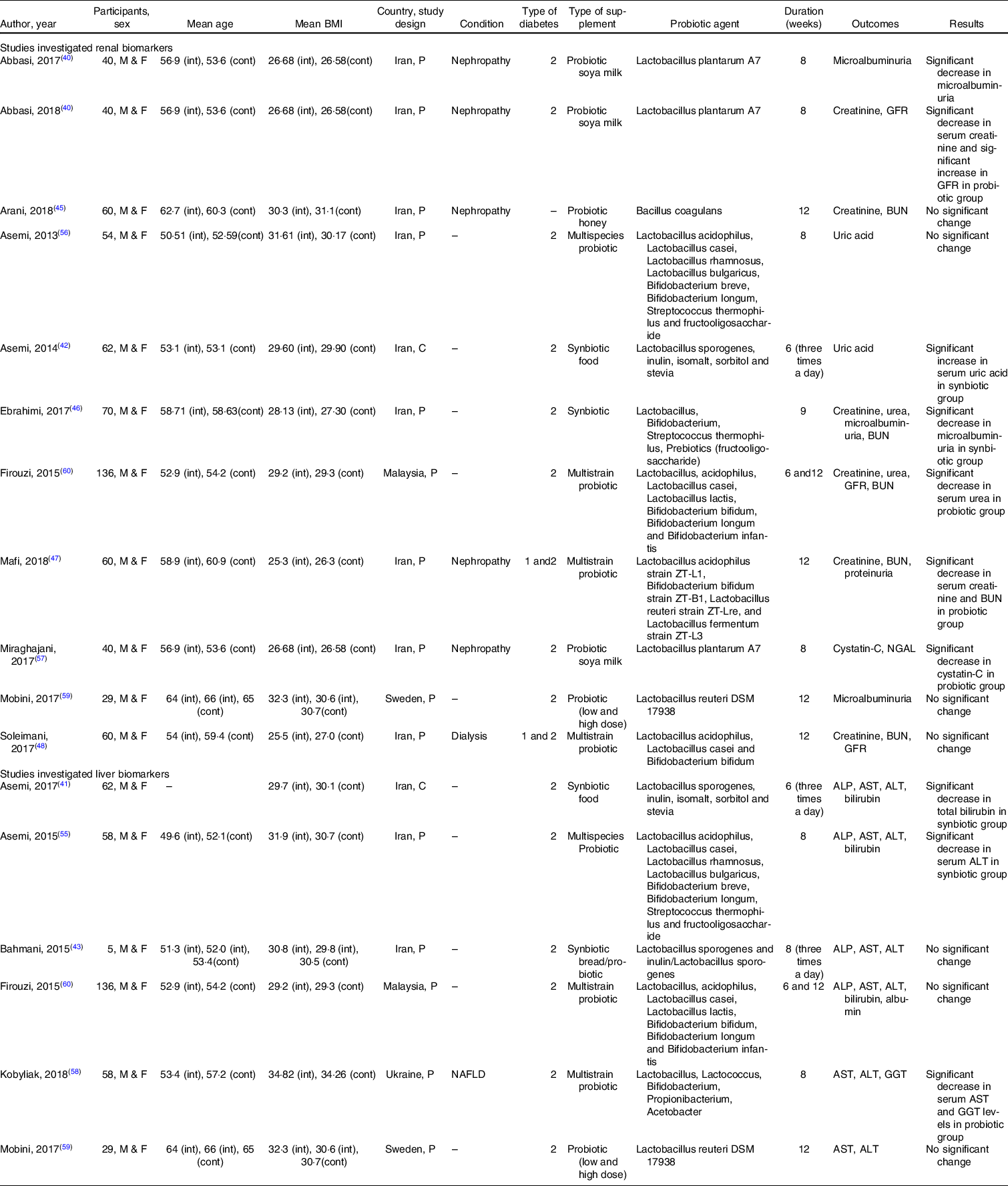
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; cont, control group; C, cross-over; F, female; GFR, glomerular filtration rate; int, intervention group; M, male; NAFLD, non-alcoholic fatty liver disease; NGAL, neutrophil gelatinase-associated lipocalin; P, parallel.
The duration of intervention ranged from 6 to 12 weeks. All the included studies administered synbiotics(Reference Ebrahimi, Nasli-Esfahani and Nadjarzade46) or probiotics(Reference Mafi, Namazi and Soleimani47,Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Asemi, Bahmani and Shakeri55,Reference Asemi, Zare and Shakeri56,Reference Kobyliak, Abenavoli and Mykhalchyshyn58–Reference Firouzi, Mohd-Yusof and Majid60) in solid pharmaceutical formulations (powder or table form), and six studies used soya milk(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Miraghajani, Zaghian and Mirlohi57) , bread(Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43), honey(Reference Arani, Emam-Djomeh and Tavakolipour45) and an unknown food containing synbiotic(Reference Asemi, Aarabi and Hajijafari41,Reference Asemi, Khorrami-Rad and Alizadeh42) as carrier. One study included two doses of probiotic, where the higher dose was considered for analysis(Reference Mobini, Tremaroli and Ståhlman59). There was also one study that presented data on synbiotic, probiotic and placebo supplementation, separately, where the probiotic in comparison with placebo was included in the analysis(Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43). Common adverse effects were reported, such as gastric disturbance(Reference Mobini, Tremaroli and Ståhlman59,Reference Firouzi, Mohd-Yusof and Majid60) , headache, hypoglycaemia and musculoskeletal symptoms(Reference Mobini, Tremaroli and Ståhlman59).
Risk of bias and quality of evidence
The Cochrane Collaboration’s tool was used to assess the methodological quality of studies. Participants, personnel and outcomes assessor were blind in all the included studies. Of the fifteen included randomised studies, two did not describe the randomisation and allocation concealment process(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Mobini, Tremaroli and Ståhlman59) . Furthermore, one study was funded partly by a non-academic source; however, the authors declared no conflict of interest, and the company did not interfere with the decision to exploit research results; therefore, we did not downgrade for funding domain(Reference Firouzi, Mohd-Yusof and Majid60). No concern was also found about incomplete data or selective reporting. Altogether, most of the included studies were rated as good quality, and two studies were fair in methodological quality(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Mobini, Tremaroli and Ståhlman59) (online Supplementary Table S3). The quality of evidence showed very low certainty for ALT, ALP, bilirubin, creatinine, GFR and microalbuminuria, and low certainty for AST and BUN (online Supplementary Table S4).
Meta-analysis
Effect of probiotics/synbiotics supplementation on liver biomarkers
Pooling data from RCT revealed probiotics/synbiotics supplementation had no significant effect on ALP(Reference Asemi, Aarabi and Hajijafari41,Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43,Reference Asemi, Zare and Shakeri56,Reference Firouzi, Mohd-Yusof and Majid60) (n 4 studies, 310 participants; weighted mean difference (WMD) = 7·26 U/L, 95 % CI –3·39, 17·91; P = 0·18; I 2 = 63·3 %; P-heterogeneity = 0·04), ALT(Reference Asemi, Aarabi and Hajijafari41,Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43,Reference Asemi, Zare and Shakeri56,Reference Kobyliak, Abenavoli and Mykhalchyshyn58–Reference Firouzi, Mohd-Yusof and Majid60) (n 6 studies, 397 participants; WMD = –0·76 U/L, 95 % CI –4·12, 2·58; P = 0·65; I 2 = 57·7 %; P-heterogeneity = 0·03), AST(Reference Asemi, Aarabi and Hajijafari41,Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43,Reference Asemi, Zare and Shakeri56,Reference Kobyliak, Abenavoli and Mykhalchyshyn58–Reference Firouzi, Mohd-Yusof and Majid60) (n 6 studies, 397 participants; WMD = –0·91 U/L, 95 % CI –3·05, 1·22; P = 0·4; I 2 = 28·1; P-heterogeneity = 0·22) and bilirubin levels (n 3 studies, 256 participants; WMD = –0·04 mg/dl, 95 % CI –0·16, 0·08; P = 0·52; I 2 = 86·2 %; P-heterogeneity = 0·001) (Fig. 2, online Supplementary Table S5). Between-study heterogeneity was moderate to high, although the small number of studies precluded a comprehensive subgroup analysis, the duration of intervention and liver complications could justify the observed heterogeneity to some extent (online Supplementary Tables S6 and S7).

Fig. 2. Forest plot of randomised controlled clinical trials illustrating weighted mean difference (WMD) in (a) ALP change (U/L), (b) ALT change (U/L), (c) AST change (U/L) and (d): bilirubin change (mg/dl) between the probiotics/synbiotics supplementation and control groups for all eligible studies. Analysis was conducted using random-effects model.
Effect of probiotics/synbiotics supplementation on renal biomarkers
Our analysis found probiotics/synbiotics supplementation reduced creatinine levels(Reference Abbasi, Mirlohi and Daniali44–Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Firouzi, Mohd-Yusof and Majid60) (n 6 studies, 426 participants; WMD = –0·10 mg/dl, 95 % CI –0·20, –0·00; P = 0·01; I 2 = 87·7 %; P-heterogeneity < 0·001), without any significant effect on GFR(Reference Abbasi, Mirlohi and Daniali44,Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Firouzi, Mohd-Yusof and Majid60) (n 3 studies, 236 participants; WMD = 4·55 ml/min per 1·73 m2, 95 % CI –0·94, 10·05; P = 0·1; I 2 = 90·7 %; P-heterogeneity < 0·001), microalbuminuria(Reference Abbasi, Ghiasvand and Mirlohi40,Reference Ebrahimi, Nasli-Esfahani and Nadjarzade46,Reference Mobini, Tremaroli and Ståhlman59) (n 3 studies, 139 participants; WMD = –10·36 Alb/Cr (mg/gr), 95 % CI –22·87, 2·16; P = 0·1; I 2 = 80·9 %; P-heterogeneity = 0·005) or BUN(Reference Arani, Emam-Djomeh and Tavakolipour45–Reference Soleimani, Zarrati Mojarrad and Bahmani48,Reference Firouzi, Mohd-Yusof and Majid60) (n 5 studies, 386 participants; WMD = –0·87 mg/dl, 95 % CI –1·91, 0·18; P = 0·1; I 2 = 36·1 %; P-heterogeneity = 0·18) (Fig. 3, online Supplementary Table S5). Subgroup analysis was performed when the number of studies was sufficient for each outcome, and the results showed a significant reduction in BUN levels when intervention lasted for 12 weeks or more (n 4 studies, 316 participants; WMD = –1·215 mg/dl, 95 % CI –1·933, –0·496; P = 0·001; I 2 = 0·0 %; P-heterogeneity = 0·41) and also showed a significant reduction in creatinine levels in patients with renal complications (n 4 studies, 220 participants; WMD = –0·209 mg/dl, 95 % CI –0·322, –0·096; P < 0·001; I 2 = 46·7 %; P-heterogeneity = 0·13). Subgroup analysis also identified duration of intervention and renal complication as the potential source of heterogeneity.

Fig. 3. Forest plot of randomised controlled clinical trials illustrating weighted mean difference (WMD) in (a) creatinine change (mg/dl), (b) GFR change (ml/min per 1·73 m2), (c) microalbuminuria change (Alb/Cr (mg/gr)) and (d): BUN change (mg/dl) between the probiotics/synbiotics supplementation and control groups for all eligible studies. Analysis was conducted using random-effects model.
Outcomes did not analyse
Uric acid
Two studies evaluated the effect of probiotic supplement and synbiotic food consumption on serum uric acid and reached to contradictory results. One study found synbiotic food supplementation significantly increased serum uric acid(Reference Asemi, Khorrami-Rad and Alizadeh42), while other study revealed no significant effect following probiotic supplementation(Reference Asemi, Zare and Shakeri56).
γ-Glutamyl transferase
One study suggested a significant 12 % decrease in serum γ-glutamyl transferase following a multistrain probiotic supplementation in type 2 diabetes patients with non-alcoholic fatty liver disease(Reference Kobyliak, Abenavoli and Mykhalchyshyn58).
Cystatin-C, neutrophil gelatinase-associated lipocalin
One study showed significant reduction in cystatin-c and marginally significant reduction in neutrophil gelatinase-associated lipocalin levels in patients with type 2 diabetic nephropathy after the consumption of probiotic soya milk compared with control(Reference Miraghajani, Zaghian and Mirlohi57).
Sensitivity analysis and publication bias
The leave-one out sensitivity analysis did not identify any study with a significant influence on the pooled effects sizes. An additional sensitivity analysis was conducted excluding the studies that examined synbiotic supplementation, and the results showed significant decreases in creatinine and BUN levels, with a significant reduction in between-study heterogeneity (online Supplementary Table S7). Publication bias was not examined due to the insufficient study for each outcome.
Discussion
This meta-analysis pooled data from RCT investigating the effect of probiotics/synbiotics supplementation on kidney and liver parameters in patients with diabetes. Our results revealed probiotics/synbiotics supplementation has no significant effect on ALT, AST, ALP, BUN, bilirubin, GFR or microalbuminuria. However, it was shown that probiotics/synbiotics may elicit beneficial effects on creatinine levels.
Emerging data indicating gut microbiota modulation by probiotic, prebiotic or synbiotic supplementation can induce favourable effects on lipid profile, glycaemic control(Reference Bock, Telo and Ramalho61) and antioxidant capacity in patients with diabetes(Reference Zheng, Guo and Jia21). It has been suggested that inflammation is the major mechanism related to diabetes complications(Reference Miraghajani, Zaghian and Mirlohi62,Reference Shahreza63) . Indeed, patients with diabetes tend to suffer from chronic inflammation, exacerbated by impaired intestinal function(Reference De Kort, Keszthelyi and Masclee64). The gut is known as a potential immune regulation gate(Reference Karakula-Juchnowicz, Rog and Juchnowicz65), and several immune, endocrine and metabolic pathways accrue between intestinal and other organs(Reference Carabotti, Scirocco and Maselli66). SCFA, the main product of gut fermentation, reduce intestinal permeability, bacteria translocation(Reference Silva, Bernardi and Frozza67) and down-regulate the expression of pro-inflammatory cytokines(Reference Sartor68). However, findings from previous meta-analysis are inconsistent(Reference Mazidi, Rezaie and Ferns69,Reference Samah, Ramasamy and Lim70) . It seems that the anti-inflammatory effects of probiotics are increased when combined with the prebiotics. Moreover, as shown in a meta-analysis, the use of synbiotics may have more beneficial effects in reducing inflammatory factors than probiotics(Reference Samah, Ramasamy and Lim70), because of the additional substrate for fermentation, and consequential growth stimulation of gut microbiota(Reference Slavin71). However, our results showed a significant reduction in creatinine and BUN levels when analysis restricted to probiotic supplementation. It may be due to the higher dose of probiotic in the studies administered probiotic, exclusively. Moreover, BUN levels improved in studies administered probiotic/synbiotic for 12 weeks or more. This association disappeared when a sensitivity analysis was conducted for studies with ≥ 8 weeks follow-up duration (data not shown). It seems, more than 12 weeks intervention may exert greater beneficial effects of probiotics. However, the number of included studies in our analysis was not enough to draw a definitive conclusion.
In line with a previous systematic review(Reference Vlachou, Ntikoudi and Govina72), we found probiotic/synbiotic supplementation may improve creatinine levels in patients with renal dysfunction, although a meta-analysis by AbdelQadir et al showed despite a significant improvement in antioxidant indices, there is no association between probiotic supplementation and creatinine, GFR or BUN levels in patients with diabetic nephropathy(Reference Abdelqadir, Hamdallah and Sibaey73). It may be contributed to misclassification of the study by Firouzi et al,(Reference Firouzi, Mohd-Yusof and Majid60) which the nephropathy was an exclusion criterion of this study, but it has been included in the analysis.
It is suggested probiotic may improve renal function through increasing anaerobic bacteria such as Lactobacillus and Bifidobacterium leading to decrease in PH and urea levels. Moreover, some probiotic species such as Bacteroides can reduce urea by their urease activity(Reference Parvez, Malik and Ah Kang74). However, our analysis found no significant association for other renal biomarkers. There is accumulating evidence suggesting some new biomarkers for kidney function, such as cystatin-C or neutrophil gelatinase-associated lipocalin, are more affected in early stages of kidney injuries than BUN or GFR(Reference Slavin71,Reference Vlachou, Ntikoudi and Govina72) . The Northern Manhattan study also indicated that cystatin-C-based GFR may be a better predictor of all-cause mortality in the elderly, in comparison with serum creatinine(Reference Abdelqadir, Hamdallah and Sibaey73). However, in our study, data were not enough to perform a meta-analysis on these predictor biomarkers.
Concordant with our findings, several previous studies showed contradictory effects of probiotic supplementation on liver enzymes in patients with diabetes(Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz43,Reference Firouzi, Mohd-Yusof and Majid60) or fatty liver diseases(Reference Mofidi, Poustchi and Yari75–Reference Loguercio, De Simone and Federico77). As a possible explanation, metformin, which was used by most of our included studied population, is known to improve lipid profile(Reference Lin, Cheng and Te Tu78), liver function(Reference Shields, Thompson and Grice79) and ovarian function(Reference Legro, Arslanian and Ehrmann80), beyond glycaemic control. It is also evident that metformin reduces micro- and macro-vascular complications and also alters gut microbiota(Reference Napolitano, Miller and Nicholls81), which may affect our results. Moreover, different probiotic strains were supplemented in included studies, and it is shown that strain variation may produce different effects on the host(Reference Boyle, Robins-Browne and Tang82,Reference Mihatsch, Braegger and Decsi83) . However, because of the small number of studies, it was not possible to assess strain-specific effects on interested outcomes. On the other hand, we assessed liver function using liver enzymes, the factors that change in the later stages of liver damage. It is suggested that standard biomarkers such as ultrasound be used in future studies.
Strengths and limitations
As far as we are aware, this is the first meta-analysis comprehensively investigating the effect of probiotics/synbiotics supplementation on kidney and liver function in patients with type 2 diabetes. However, one previous meta-analysis study investigated the effect of probiotic supplementation on kidney function in patients with diabetic nephropathy, with non-significant results(Reference Abdelqadir, Hamdallah and Sibaey73). Pooling data from good quality RCT permits causal associations to be drawn; however, there are some considerable limitations. First, the number of included studies was small for each outcome, which affects the validity of the results. Second, there was varied setting among studies, which made it difficult to assess the isolate effect of probiotic supplementation on the outcomes, including probiotic species, probiotic carrier, the medication used and body weight. Third, although macronutrients intake was controlled in most of the included studies, fibre intake or antioxidant nutrients (such as vitamin E, C, D or n-3) were not considered in analyses. Fourth, renal and liver biomarkers in most of the included studies were secondary outcomes; therefore, the studies may not have an adequate sample size to detect a significant association. Fifth, none of the included studies used gold standard biomarkers, resulting reduced validity of the results. Sixth, the absence of any information on the composition of colon microbiota after the intervention with probiotics/synbiotics makes it difficult to draw conclusions about the effect of the supplement on changing the gut microbiota, which is suggested to be studied in future researches. Seventh, the certainty of evidence was low or very low; as, most of the included participants were from same location (Iran), and the point estimate was smaller than 5 % baseline value of interested outcomes, leading to downgrading for inconsistency and imprecision, respectively.
Conclusion
In the present systematic review and meta-analysis, we assessed the effects of probiotics/synbiotic treatment on the liver and kidney biomarkers in patients with T2DM. The results of our meta-analysis indicated that probiotics/synbiotic treatment may reduce creatinine levels. However, due to the very low certainty of evidence, more clinical data using gold standard biomarkers are needed, globally, to clarify the role of probiotics, the most beneficial bacteria and the optimal dosage in T2DM patients.
Acknowledgements
The authors wish to thank the Shahid Sadoughi University of Medical Sciences Librarians for their assistance with database searches.
This work was funded by the North Khorasan University of Medical Sciences which is thanked for their financial support (4000027).
S. A. and S. S. designed the review; F. M. and S. S. conducted the major database search according to search strategy; F. M. and S. S. did the data extraction; S. S. performed the analysis; S. A. wrote the manuscript’s draft; and all authors evaluated the final version of the manuscript precisely and approved it.
The authors report no conflict of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521003780










