Fe, an essential micronutrient, is involved in redox reactions and plays an integral role in various physiological functions( Reference Huang 1 ). However, excess Fe can also cause tissue damage, disrupt cell processes and components through its pro-oxidative effects and exacerbate the progression of diseases such as cancer through the generation of reactive oxidative species( Reference Toyokuni 2 – Reference Padmanabhan, Brookes and Iqbal 4 ). There are two major forms of dietary Fe: non-haem and haem Fe. Non-haem Fe, the major type of food Fe, is found in plants, meat and supplements. Haem Fe is a small proportion of the total Fe consumed and is found primarily in red meat( Reference Carpenter and Mahoney 5 ). On average, haem Fe is better absorbed than non-haem Fe( Reference Oates and West 6 ). It has been reported that different forms of Fe (i.e. haem v. non-haem) and sources of Fe (i.e. red meat v. plants) may contribute differently to colorectal cancer risk( Reference Cross, Ferrucci and Risch 7 , Reference Tseng, Sandler and Greenberg 8 ).
In 2017, the World Cancer Research Fund/American Institute for Cancer Research reported that there is some evidence that consumption of foods containing haem Fe might increase the risk of colorectal cancer( 9 ). Epidemiological studies have reported no association( Reference Ruder, Berndt and Gilsing 10 – Reference Kato, Dnistrian and Schwartz 17 ), positive association( Reference Senesse, Meance and Cottet 18 – Reference Sun, Zhu and Wang 22 ) and negative association( Reference Cross, Ferrucci and Risch 7 ) between total dietary Fe and colorectal cancer risk. Furthermore, some studies( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 , Reference Zhang, Giovannucci and Smith-Warner 13 , Reference Kabat, Miller and Jain 14 , Reference Balder, Vogel and Jansen 16 , Reference Bastide, Pierre and Corpet 23 – Reference Hara, Sasazuki and Inoue 26 ) have explored the association between specific types of Fe intake and colorectal cancer risk. Four( Reference Cross, Ferrucci and Risch 7 , Reference Bastide, Pierre and Corpet 23 – Reference Larsson, Adami and Giovannucci 25 ) of the above-mentioned studies reported positive association of haem Fe intake with colorectal cancer risk. Only one study assessed Fe from meat intake in association with colorectal cancer risk and no significant association was found( Reference Kabat, Miller and Jain 14 ). In addition, alcohol consumption is known to disrupt Fe homoeostasis( Reference Leggett, Brown and Bryant 27 – Reference Milman, Byg and Ovesen 29 ) through transiently generating free Fe which is carcinogenic( Reference McCord 30 , Reference Linn 31 ). Some studies have indicated that the association of colorectal cancer with haem Fe intake is more pronounced among alcohol drinkers( Reference Lee, Anderson and Harnack 24 , Reference Larsson, Adami and Giovannucci 25 ).
So far, most of the epidemiological studies on the relationship between Fe intake and colorectal cancer risk have been conducted in the Western countries, in which dietary habits were different from the Chinese populations( Reference Zhang, Shu and Si 32 ). To the best of our knowledge, no study has reported the association between the consumption of various sources of Fe and the risk of colorectal cancer. Therefore, the present case–control study specifically evaluated the association between the daily intake of dietary Fe from different food sources and forms and the risk of colorectal cancer in a Chinese population residing in Guangdong Province. We also examined whether the associations between Fe intake and colorectal cancer risk were modified by alcohol consumption.
Methods
Study subjects
This ongoing case–control study which began in July 2010 has been reported previously( Reference Zhong, Fang and Pan 33 ). Briefly, case subjects aged 30–75 years were consecutively recruited from the surgical units of the Sun Yat-sen University Cancer Center, Guangzhou, China. Eligible criteria for cases included that patients with incident, first primary, histologically confirmed colorectal cancer diagnosed no more than 3 months before the recruitment interview, and that cases were either natives of Guangdong Province or had lived in Guangdong for more than 5 years. We excluded the patients if they could not speak or understand Mandarin/Cantonese. From July 2010 to November 2017, a total of 2409 eligible cases were identified and 2157 were successfully interviewed, with a response rate of 89·53 %. Of them, 252 patients did not complete the investigation mainly due to fatigue, communication barriers and refusal. Moreover, subjects with an energy intake that was too low or too high (<2510 or >14 644 kJ/d (<600 or >3500 kcal/d) for female and <3347 or >17 573 kJ/d (<800 or >4200 kcal/d) for male)( Reference Nimptsch, Zhang and Cassidy 34 ) were not included in the analysis. Nineteen subjects with an energy intake that was too low or too high were excluded. Finally, 2138 cases were included in the analysis.
Two control groups were used in this case–control study. The control subjects were frequency matched to cases by 5-year age group and sex. The inclusion criteria for the controls were the same as those for the cases. The first control group was recruited from the inpatients admitted to the Departments of Otorhinolaryngology, Plastic and Reconstructive Surgery and Vascular Surgery in the First-affiliated Hospital of Sun Yat-sen University, during the same time period. They mainly suffered from chronic otitis media, chronic sinusitis, sudden deafness, trigeminal neuralgia, varicose veins, vocal cord polyp, orthopaedics and facial paralysis. So far, no evidence has been found that these conditions from the above-mentioned diseases were obviously related to a dietary cause. Finally, 1054 hospital-derived controls were identified and 920 were successfully interviewed, yielding a participation rate of 87·29 %. The other control group was recruited from residents in the same community via written invitations, advertisements or referrals. The eligibility criteria for the controls were the same as described for the cases except that they had no prior history of any cancer. A total of 1224 controls from the community were successfully interviewed and included in the study.
We assumed that people with higher dietary Fe intake represented 25 % of the general population, the estimated OR between the consumption of total dietary Fe and colorectal cancer risk was 1·26( Reference Ruder, Berndt and Gilsing 10 ), the type I error rate was <0·05 (α=0·05), the power of test was 90 % (β=0·10) and the response rate was 90 %. Based on these assumptions, we require a sample size of 1988 cases.
This study was conducted according to the guidelines of Declaration of Helsinki. The procedures and protocols of this study were approved by the Ethical Committee of School of Public Health, Sun Yat-sen University. Written requirements of this study were obtained from all participants.
Data collection
Data were collected by trained interviewers through face-to-face interviews. A structured questionnaire was used to collect information on socio-demographic characteristics, body weight and height, lifestyle factors (e.g. active and passive smoking, alcohol consumption and physical activity) and family history of cancer. Information on the uses of nutritional supplement was also obtained. The questionnaire also included the cooking method (deep-frying and pan-frying), frequency and degree of frying using questions on the appearance of the surface of prepared meat, fish and egg at increasing levels of doneness. For female subjects, menstrual and reproductive histories were also acquired. Relevant medical diagnoses and pathological findings were gained from the medical records. BMI was calculated as the ratio of weight (kg) to squared height (m2). In this study, those who had ever smoked were defined as smoking at least 1 cigarette/d for more than six consecutive months. Passive smokers were defined as being exposed to the smoke exhaled for at least 5 min/d during the previous 5 years. Regular drinking was defined as drinking alcohol at least once per week over the past year. Nutritional supplements (e.g. Ca and multivitamin) users were defined as having taken the relevant pills for at least 3 months. Postmenopausal status was defined as at least 12 months since the last menstrual cycle. Physical activity intensity was evaluated by the interviewers based on self-reported occupational, household and recreational physical activities in the past year. Frequency (d/week) and duration (h/d) data were gained for household and leisure activities. Occupational activity was classified by labour intensity as follows: (a) not working, (b) long-time sitting, (c) low intensity, (d) moderate intensity or (e) vigorous intensity, with examples provided. Information on household and recreational physical activities was also collected in this study. It was categorised into light physical activity (e.g. walking), moderate physical activity (e.g. jogging, mountaineering, playing table tennis) and vigorous physical activity (e.g. running, playing football/basketball). The mean metabolic equivalent task (MET) hours value of each activity was obtained by estimating the average of all comparable activities in the Compendium of Physical Activities(
Reference Ainsworth, Haskell and Whitt
35
,
Reference Ainsworth, Haskell and Herrmann
36
). MET-h/week over the past 12 months was calculated as follows:
![]() $$\eqalignno{ &\hskip-58pt {\rm Number \;of \;d\,/\,week {\times}number \;of \;h\,/\,d{\times}} \cr & \hskip-58pt {\rm MET \;of \;a \;specific \;type \;of \;activity{\equals}MET\hbox{-}h\,/\,week}{\rm .}$$
$$\eqalignno{ &\hskip-58pt {\rm Number \;of \;d\,/\,week {\times}number \;of \;h\,/\,d{\times}} \cr & \hskip-58pt {\rm MET \;of \;a \;specific \;type \;of \;activity{\equals}MET\hbox{-}h\,/\,week}{\rm .}$$
Measurement of dietary exposure
An eighty-one-item FFQ( Reference Zhang and Ho 37 ) was used to estimate study subjects’ dietary intake. Information on the frequency of intake and portion size during 12 months before diagnosis for cases and interview for controls was collected. It was used to estimate the average intake of each food item in g/d. Photographs of commonly consumed foods were available to help quantify portion sizes. The main food groups included cereal products, legumes, vegetables, fruits, red and processed meat, poultry, fish, egg, dairy products and nuts. For this analysis, plants included cereal, legumes, vegetables and fruits. Meats were grouped into red meat and white meat. Red meat consisted of the following food group items: pork, beef, lamb, organ meat and processed meat. White meat primarily constituted poultry and fish. Poultry included chickens, ducks and geese. Fish included freshwater fish, saltwater fish, canned fish, salted fish, crab, shrimp, prawn, squid, cuttle, scallops, mussel and whelk. The energy and nutrient intakes were calculated according to the 2002 Chinese Food Composition Table( Reference Yang, Wang and Pan 38 ).
Totally, seven categories of Fe intakes, consisting of two major forms of Fe and four different food sources of Fe, were examined. This included total dietary Fe, haem Fe, non-haem Fe, Fe from plants, Fe from meat, Fe from white meat and Fe from red meat. Haem Fe intake was calculated by two methods, using different proportions of haem Fe from different types of meat: 65 % for beef, 39 % for pork and pork products (such as ham, bacon and luncheon meats), 26 % for chicken and fish, 21 % for liver( Reference Balder, Vogel and Jansen 16 ) and, alternatively, using 40 % as the average proportion of haem Fe in all meats( Reference Monsen and Balintfy 39 ). Since the results were similar for both methods, data of haem Fe intake were presented using the first method. In the present study, Fe from dietary supplements was not calculated as part of the exposure.
The validity and reproducibility of the FFQ, with six 3-d energy-adjusted diet records, have been confirmed among the local population. The Pearson’s correlation coefficients between the FFQ and six 3-d dietary records were 0·25–0·65 for nutrients and 0·30–0·68 for food groups( Reference Zhang and Ho 37 ).
Statistical analysis
All data analyses were conducted using SPSS 20.0 (SPSS Inc.). We used the t test or Wilcoxon signed-rank test to evaluate the difference in the continuous variables between the cases and controls and used the χ 2 test for the categorical variables. The dietary intake data were adjusted for total energy intake through the residual method( Reference Willett, Howe and Kushi 40 ). Dietary Fe intake were categorised into quartiles (Q1–Q4) based on the distribution among the controls. Multivariable logistic regression models were used to estimate the OR and 95 % CI for the associations between dietary Fe intake and colorectal cancer risk, with the lowest quartile as the reference group. Tests for trend were performed by entering the categorical variables (Q1–Q4) as continuous variables in the regression models. Spearman’s correlation coefficients were calculated to examine the correlations between total dietary Fe, different forms of Fe and sources of Fe.
Several potential confounders were included in multivariable models based on comparison of the baseline characteristics of the cases and controls or previous reported confounders. Confounding variables included age (continuous), sex (male/female), residence (urban/rural), marital status (married/others), educational level (primary school or below/junior high school/senior high school or secondary technical school/college or above), occupation (administrator or other white-collar worker/blue-collar worker/farmer or others), income (<2000/2001–5000/5001–8000/>8001 yuan/month, Renminbi), occupational activity (not working/sedentary/light occupation/moderate occupation/heavy activity occupation), household and recreational physical activities (continuous), first-degree relative with cancer (yes/no), smoking status (current/never or past), passive smoking (yes/no), alcohol consumption (yes/no), nutritional supplement use (yes/no), deep-fried/pan-fried cooking method user (yes/no) and BMI (continuous). In females, age at menarche (continuous) and menopausal status (premenopausal/postmenopausal) were also adjusted. Stratified analysis by sex and alcohol consumption and subgroup analysis by cancer site (colon or rectal cancer) were conducted. The interaction between sex, alcohol consumption and Fe intake in relation to colorectal cancer risk was evaluated by multivariable logistic regression. We also examined possible heterogeneity in the association between different forms of Fe, sources of Fe and colorectal cancer according to cancer subsite using a polytomous logistic regression model. To calculate the P heterogeneity between colorectal cancer sites, we used likelihood ratio test comparing the model in which the association with different types of Fe was allowed to vary by cancer site (colon or rectal cancer) to a model in which a common association was assumed( Reference Wang, Spiegelman and Kuchiba 41 ). In the present study, all P values were two sided and P<0·05 was deemed to indicate statistical significance.
Results
Among the 2138 cases, 1219 were males and 919 were females. In total, 1303 cases were diagnosed with colon cancer and 829 were diagnosed with rectal cancer, six were unclear of its cancer site. Five female cases had missing data about age at menarche, and we filled them with mean menarche age of the cases.
The socio-demographic characteristics of the study participants are presented in Table 1. Compared with the controls, cases tended to live in rural area, to be married, had less education, lower income, heavier occupational activities and less household and recreational physical activities and had lower BMI. Cases were also less likely to use nutritional supplements, had higher frequency of smoking, passive smoking, regular alcohol consumption, using deep-fried/pan-fried cooking method, and had family history of cancer compared with controls. More female colorectal cancer cases were premenopausal and had later age at menarche compared with controls.
Table 1 Socio-demographic characteristics and selected risk factors of colorectal cancer in the study populationFootnote * (Numbers and percentages; mean values and standard deviations)
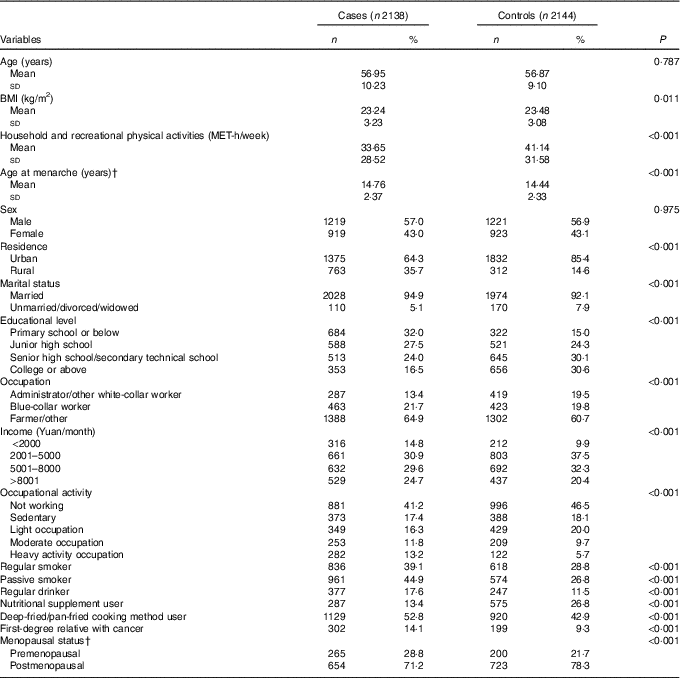
MET, metabolic equivalent task.
* Continuous variables were evaluated using t tests or Wilcoxon rank-sum tests. Categorical variables were evaluated using χ 2 tests.
† Among female subgroup.
The median daily consumption of dietary Fe is shown in Table 2. Compared with the controls, cases had a higher intake of haem Fe, Fe from meat, Fe from red meat, total meat and red meat. However, cases had a lower intake of total energy, Fe from plants, Fe from white meat, white meat, poultry and fish compared with controls. A borderline higher intake of total dietary Fe was also detected among cases (P=0·062). Between cases and controls, no significant difference was found in non-haem Fe. Except for non-haem Fe with haem Fe and Fe from red meat, total dietary Fe, haem Fe, non-haem Fe and different sources of Fe were all significantly correlated with each other. The Spearman’s correlation coefficients ranged from 0·006 to 0·974 (‘Appendix’).
Table 2 Intakes of energy, total dietary iron and different types of iron among cases and controlsFootnote * (Mean values; medians; 25th and 75th percentiles)
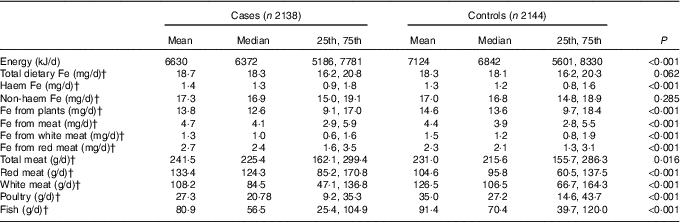
* Wilcoxon rank-sum test comparing the median consumption levels between cases and controls.
† Consumption was adjusted for total energy intake by the residual method.
Associations between different types of dietary Fe intake and colorectal cancer risk are presented in Table 3. Intake of Fe from plants and Fe from white meat were inversely associated with colorectal cancer risk, whereas intake of haem Fe and Fe from red meat were positively associated with colorectal cancer risk. After adjusting for the potential confounders, the multivariable OR for the highest quartile compared with the lowest quartile were 0·72 (95 % CI 0·59, 0·87, P trend<0·001) for Fe from plants, 0·54 (95 % CI 0·45, 0·66, P trend<0·001) for Fe from white meat, 1·26 (95 % CI 1·04, 1·53, P trend=0·005) for haem Fe and 1·83 (95 % CI 1·49, 2·24, P trend<0·001) for Fe from red meat intake, respectively. However, total dietary Fe, non-haem Fe and Fe from meat displayed no significant association with colorectal cancer risk. The adjusted OR were 1·16 (95 % CI 0·96, 1·41, P trend=0·275) for total dietary Fe, 1·07 (95 % CI 0·89, 1·30, P trend=0·646) for non-haem Fe and 1·11 (95 % CI 0·91, 1·35, P trend=0·094) for Fe from meat, respectively.
Table 3 Colorectal cancer according to quartiles (Q) of different types of iron (Odds ratios and 95 % confidence intervals)
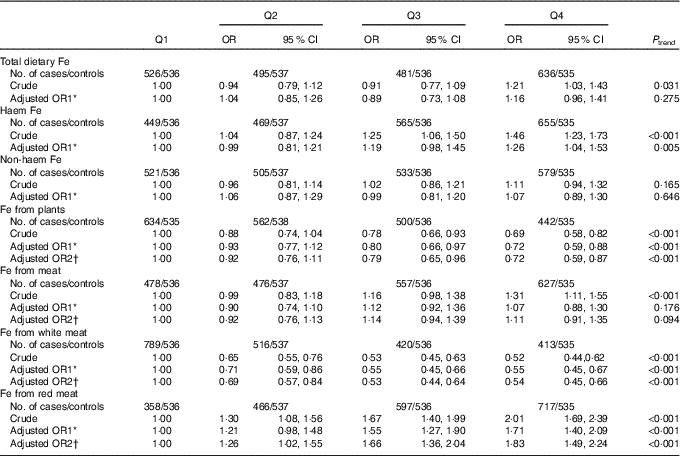
* OR1 adjusted for age, sex, residence, marital status, educational level, occupation, income, occupational activity, household and recreational physical activities, first-degree relative with cancer, smoking status, passive smoking, alcohol consumption, nutritional supplement use, deep-fried/pan-fried cooking method use and BMI.
† OR2 adjusted for above confounders, and mutually adjusted for each other: ‘Fe from plants’ additionally adjusted for ‘Fe from meat’, ‘Fe from meat’ additionally adjusted for ‘Fe from plants’, ‘Fe from white meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from red meat’, ‘Fe from red meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from white meat’.
In the stratified analyses, there was no significant interaction in the associations between different categories of Fe intake and colorectal cancer risk modified by alcohol consumption (P interaction>0·05) (Table 4). Stratified analysis by sex indicated that Fe from white meat intake was inversely associated with colorectal cancer risk in both sexes, and no significant association was found between total dietary Fe, non-haem Fe and colorectal cancer risk in both males and females. However, Fe from plant intake was found to be inversely associated with colorectal cancer risk only among males (P interaction<0·001), with an adjusted OR of 0·46 (95 % CI 0·36, 0·60, P trend<0·001) comparing the highest with the lowest quartile. Higher intakes of haem Fe, Fe from meat and Fe from red meat were only found to be associated with the increased risk of colorectal cancer among males but not among females (Table 5).
Table 4 Colorectal cancer according to quartiles (Q) of different types of iron by alcohol consumption (Odds ratios and 95 % confidence intervals)
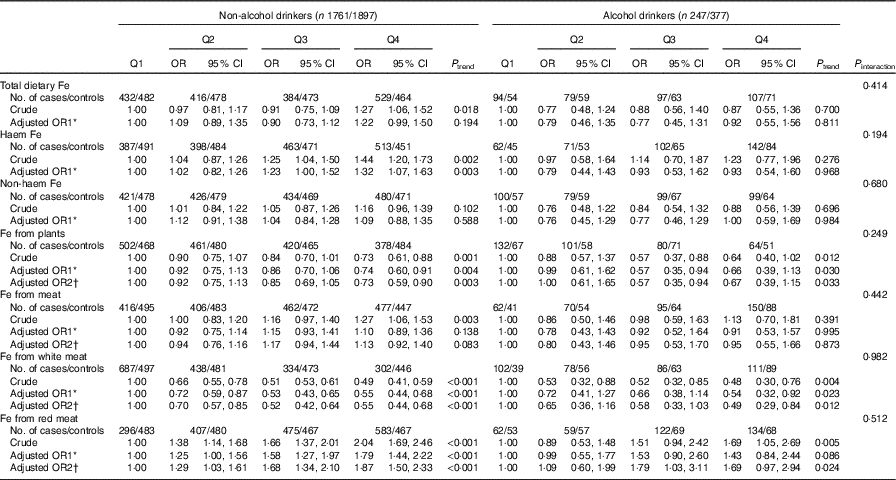
* OR1 adjusted for age, sex, residence, marital status, educational level, occupation, income, occupational activity, household and recreational physical activities, first-degree relative with cancer, smoking status, passive smoking, nutritional supplement use, deep-fried/pan-fried cooking method use and BMI.
† OR2 adjusted for above confounders, and mutually adjusted for each other: ‘Fe from plants’ additionally adjusted for ‘Fe from meat’, ‘Fe from meat’ additionally adjusted for ‘Fe from plants’, ‘Fe from white meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from red meat’ , ‘Fe from red meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from white meat’.
Table 5 Colorectal cancer according to quartiles (Q) of different types of iron by sex (Odds ratios and 95 % confidence intervals)
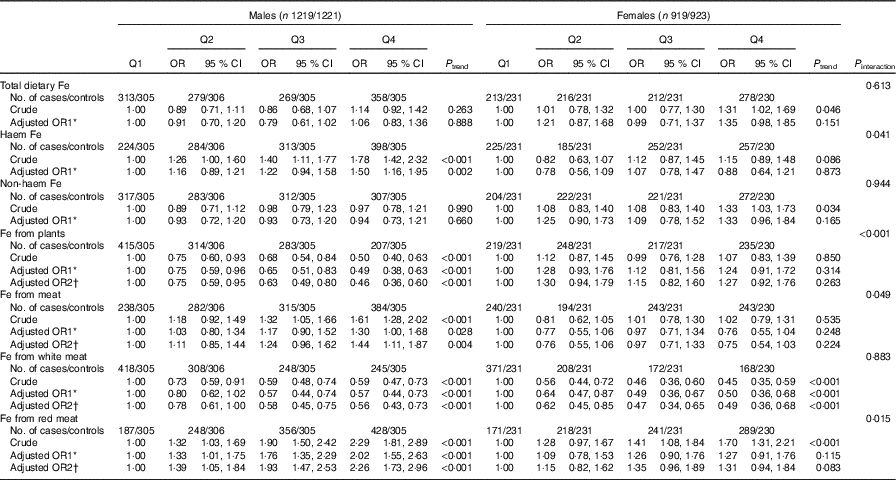
* OR1 adjusted for age, residence, marital status, educational level, occupation, income, occupational activity, household and recreational physical activities, first-degree relative with cancer, smoking status, passive smoking, alcohol consumption nutritional supplement use, deep-fried/pan-fried cooking method use and BMI. Female subjects additionally adjusted for age at menarche and menopausal status.
† OR2 adjusted for above confounders, and mutually adjusted for each other: ‘Fe from plants’ additionally adjusted for ‘Fe from meat’, ‘Fe from meat’ additionally adjusted for ‘Fe from plants’, ‘Fe from white meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from red meat’, ‘Fe from red meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from white meat’. Female subjects additionally adjusted for age at menarche and menopausal status.
The results of the subgroup analysis by cancer site are presented in Table 6. Except for non-haem Fe, there was no evidence of differences by cancer site for different categories of Fe intake (P heterogeneity>0·05). Non-haem Fe intake was non-significantly positively associated with colon cancer risk but non-significantly inversely associated with rectal cancer risk, with adjusted OR of 1·19 (95 % CI 0·95, 1·48) and 0·96 (95 % CI 0·75, 1·24), respectively, comparing the highest with the lowest quartile (P heterogeneity=0·035). The dose–response associations were found between Fe from meat, haem Fe and colon cancer risk (P trend=0·003 and P trend=0·001, respectively) but not with rectal cancer (P trend=0·952 and P trend=0·141, respectively), although the formal tests for heterogeneity of effect between the two sites were not statistically significant (P heterogeneity=0·132 and P heterogeneity=0·734, respectively).
Table 6 Associations between different types of iron and colorectal subsites (colon and rectum) (Odds ratios and 95 % confidence intervals)
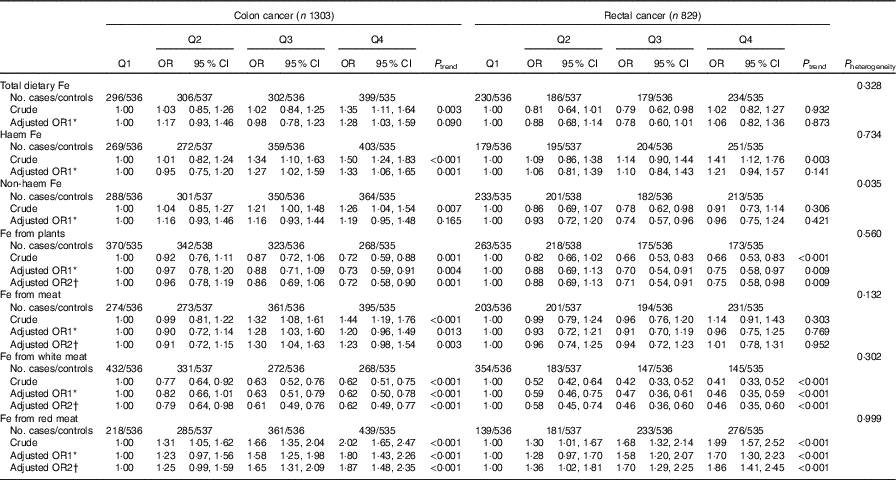
Q, quartiles.
* OR1 adjusted for age, sex, residence, marital status, educational level, occupation, income, occupational activity, household and recreational physical activities, first-degree relative with cancer, smoking status, passive smoking, alcohol consumption, nutritional supplement use, deep-fried/pan-fried cooking method use and BMI.
† OR2 adjusted for above confounders, and mutually adjusted for each other: ‘Fe from plants’ additionally adjusted for ‘Fe from meat’, ‘Fe from meat’ additionally adjusted for ‘Fe from plants’, ‘Fe from white meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from red meat’, ‘Fe from red meat’ additionally adjusted for ‘Fe from plants’ and ‘Fe from white meat’.
Discussion
This hospital-based case–control study showed that intake of Fe from plants, Fe from white meat were inversely associated with colorectal cancer risk. Higher intake of haem Fe and Fe from red meat were positively associated with the risk of colorectal cancer. However, total dietary Fe, non-haem Fe and Fe from meat were not found to be related to colorectal cancer risk.
The present study observed a positive association between haem Fe intake and colorectal cancer risk. Consistent with this result, three( Reference Cross, Ferrucci and Risch 7 , Reference Lee, Anderson and Harnack 24 , Reference Larsson, Adami and Giovannucci 25 ) of eight( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 , Reference Zhang, Giovannucci and Smith-Warner 13 , Reference Kabat, Miller and Jain 14 , Reference Balder, Vogel and Jansen 16 , Reference Lee, Anderson and Harnack 24 – Reference Hara, Sasazuki and Inoue 26 ) cohort studies examining the relationship between haem Fe intake and colorectal cancer risk reported a positive association between haem Fe intake and colorectal cancer risk. Moreover, a meta-analysis consisting of five prospective studies showed that higher haem Fe intake was associated with an 18 % increased risk of colon cancer( Reference Bastide, Pierre and Corpet 23 ). These inconclusive results might be due to the following reasons. First, the main sources of haem Fe, total meat and different kinds of meat consumption were different among different populations( Reference Oude, Seferidi and Stamler 42 ), which led to the different levels of haem Fe intake. In the present study, the mean consumption of total meat, red meat and white meat among controls were 231·0, 104·6 and 126·5 g/d, respectively. This result was consistent with Nutritional Survey in Guangdong Province (221·5 g/d total meat, 118·5 g/d red meat and 103 g/d white meat)( Reference Zhang, Ma and Chen 43 ). Beef intake, accounting for 78 %( Reference Sui, Raubenheimer and Cunningham 44 ), 72 %( Reference Carvalho, Selem and Miranda 45 ) and 36 %( Reference Egeberg, Olsen and Christensen 46 ) of red meat consumed in Australia, Brazil and the Denmark, respectively, was much higher than that in the residents of Guangdong Province (accounting for 7–8 % of red meat)( Reference Zhang, Ma and Chen 43 ). Haem Fe is mainly derived from red meat, especially beef, with 65 % haem Fe in beef( Reference Balder, Vogel and Jansen 16 ). Therefore, the median energy-adjusted haem Fe intake in the present study (1·2 mg/d) was lower than that in Canadians (1·99–2·40 mg/d)( Reference Kabat, Miller and Jain 14 ) and Americans (1·37 mg/d)( Reference Lee, Folsom and Jacobs 47 ). Moreover, although a similar level of fish intake (90 g/d for males and 86 g/d for females) was reported in Japan( Reference Kurotani, Nanri and Goto 48 ) with the present study (mean intake of 91·4 g/d), due to a lower red meat intake in Japanese (45·6 g/d)( Reference Kurotani, Nanri and Goto 48 ), haem Fe intake was much lower (0·5 mg/d) in Japanese( Reference Hara, Sasazuki and Inoue 26 ) than that in the present study. Second, the variation in the quantity of haem Fe caused by cooking methods can partly explain the difference. Haem Fe can be partially converted to non-haem Fe depending on the type and extent of the cooking method( Reference Sinha, Cross and Curtin 49 ). Compared with Western countries, deep-frying is the most common cooking method for meat and higher degree of meat doneness was made in the studied population( Reference Xu, Dai and Xiang 50 ). Third, the way of calculating dietary haem Fe differed in different studies. The most common method was to calculate haem Fe content in the diet using 40 % as the average proportion of Fe in all meats( Reference Kabat, Miller and Jain 14 , Reference Lee, Anderson and Harnack 24 , Reference Larsson, Adami and Giovannucci 25 ). The second method was based on different proportions of Fe from different types of meat, such as 65 % for beef, 39 % for pork and pork products, 26 % for chicken and fish and 21 % for liver( Reference Kabat, Miller and Jain 14 , Reference Balder, Vogel and Jansen 16 ). The third one was to use a haem Fe database( Reference Cross, Harnly and Ferrucci 51 ) based on a detailed meat cooking questionnaire( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 ). The present study found that higher intake of haem Fe were positively associated with colorectal cancer risk using the first and second methods. However, two studies using the second method did not find an association between haem Fe intake and colorectal cancer risk( Reference Kabat, Miller and Jain 14 , Reference Balder, Vogel and Jansen 16 ). One( Reference Cross, Ferrucci and Risch 7 ) of the two studies( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 ) using the third method, haem database, reported a significant positive association between haem Fe and colorectal cancer risk.
Consistent with our result, one female cohort study conducted in Canada demonstrated a null association between non-haem Fe intake and colorectal cancer risk( Reference Kabat, Miller and Jain 14 ). It has been reported that non-haem Fe may exert distinct effect on colorectal cancer compared with haem Fe( Reference Cross, Ferrucci and Risch 7 ). Generally, non-haem Fe is less absorbed from the diet. On average, adult men and women absorb 6 and 13 % of dietary non-haem Fe, respectively( Reference Hallberg 52 ). Unabsorbed non-haem Fe from the diet travels through the alimentary tract, eventually ending up in the colon and rectum before it is lost in the faeces. It may interact with other unabsorbed food components, waste and then impact colorectal carcinogenesis( Reference Nelson 53 ). More attentions are needed to clarify the association between non-haem Fe and colorectal cancer risk.
Previous studies have highlighted the importance of distinguishing between different sources of Fe( Reference Tseng, Sandler and Greenberg 8 ). Therefore, the present study further explored the association between sources of Fe from meat and plants and colorectal cancer risk. The results demonstrated no significant association between Fe from meat and colorectal cancer risk. However, the present study found the positive association between Fe from red meat intake and colorectal cancer risk, and inverse association between Fe from white meat and colorectal cancer risk. So far, only two studies assessed the risk of colorectal neoplasm in association with meat Fe intake( Reference Kabat, Miller and Jain 14 , Reference Ferrucci, Sinha and Graubard 54 ). Consistent with our findings, both studies found no significant association between colorectal neoplasm and Fe from meat. No study has yet investigated the association between various components of meat Fe and colorectal cancer risk. One of the main differences between red and white meat is the haem Fe content. White meat contains less haem Fe, probably 26 %( Reference Balder, Vogel and Jansen 16 ). This might help to account for the inverse association between intake of Fe from white meat and colorectal cancer risk observed in the present study. Moreover, the inverse relationship between Fe from white meat and the risk of colorectal cancer may partly be due to other contents present in white meat along with Fe, such as n-3 PUFA and vitamin D in some species of fish, which may protect against colorectal cancer( Reference Zhong, Fang and Pan 55 , Reference Hartman, Albert and Snyder 56 ).
The present study provided the evidence for the decreased risk of colorectal cancer in association with high dietary intake of plant-derived Fe. So far, the association between plant-derived Fe and the risk of colorectal cancer has not yet been clearly explored. Plant-derived Fe primarily consisted of Fe from vegetables, fruits and grains in the studied population. Tseng et al. ( Reference Tseng, Sandler and Greenberg 8 ) noted that dietary Fe may be beneficial if it is derived from fruits and vegetable sources as opposed to meat. In addition, Cross et al. ( Reference Cross, Ferrucci and Risch 7 ) pointed out that various sources of dietary Fe are generally healthy (e.g. fruit juice, fortified cereals, bread).
The findings regarding total dietary Fe and colorectal cancer risk are inconsistent. Four( Reference Senesse, Meance and Cottet 18 – Reference Deneo-Pellegrini, De Stefani and Boffetta 20 , Reference Sun, Zhu and Wang 22 ) of five( Reference van Lee, Heyworth and McNaughton 12 , Reference Senesse, Meance and Cottet 18 – Reference Deneo-Pellegrini, De Stefani and Boffetta 20 , Reference Sun, Zhu and Wang 22 ) case–control studies revealed an increased risk of colorectal cancer with higher consumption of total dietary Fe. However, another case–control study, including 854 cases and 958 controls, found a null association between dietary Fe and colorectal cancer( Reference van Lee, Heyworth and McNaughton 12 ). So far, seven prospective studies evaluating the relationship between Fe intake and colorectal cancer risk have been published( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 , Reference Zhang, Giovannucci and Smith-Warner 13 – Reference Balder, Vogel and Jansen 16 , Reference Wurzelmann, Silver and Schreinemachers 21 ). Except for two prospective studies( Reference Cross, Ferrucci and Risch 7 , Reference Wurzelmann, Silver and Schreinemachers 21 ), the remaining five studies showed a non-significant relationship between dietary Fe and colorectal cancer incidence( Reference Ruder, Berndt and Gilsing 10 , Reference Zhang, Giovannucci and Smith-Warner 13 – Reference Balder, Vogel and Jansen 16 ). Furthermore, a recent pooled analysis of seven prospective studies in the United Kingdom Dietary Cohort Consortium observed no significant association between total dietary Fe intake and colorectal cancer risk( Reference Key, Appleby and Masset 11 ). Consistent with these studies, the present study did not observe a statistically significant association between total dietary Fe intake and colorectal cancer risk. The conflicting results may be due in part to the various composition of total dietary Fe among different populations. As reported, diverse components of dietary Fe may contribute to the distinct effect on colorectal cancer( Reference Cross, Ferrucci and Risch 7 ). Haem Fe accounted for only 7 % of the total Fe in the present study, in contrast to taking up more than 16 % in the USA( Reference Kallianpur, Lee and Gao 57 ). Moreover, two studies( Reference Cross, Ferrucci and Risch 7 , Reference Ruder, Berndt and Gilsing 10 ) in the USA and one study in Canada( Reference Sun, Zhu and Wang 22 ) calculated the total Fe by including both dietary and supplements. These studies reported higher total Fe intake (26·6( Reference Ruder, Berndt and Gilsing 10 ) or 21·5 mg/d( Reference Cross, Ferrucci and Risch 7 ) in the USA and 24·5 mg/d in Canada( Reference Sun, Zhu and Wang 22 )) than that in the present study without including Fe from supplements (mean energy-adjusted total dietary Fe of 18·3 mg/d). In addition, the use of Fe-fortified foods in Chinese adults, such as Fe-fortified cereals or soya sauce, is <5 %( Reference Li, Sun and Zhao 58 , Reference Qing and Tang 59 ). However, in the USA, it was reported that more than 50 % of its industrially milled maize flour was fortified with Fe( 60 ). Furthermore, compared to the Western countries, tea, phytates and legumes, which are significant inhibitors of Fe absorption, were highly consumed in Chinese population( Reference Kallianpur, Lee and Gao 57 ). These differences may make it hard to detect the association of total dietary Fe with colorectal cancer risk in our study.
In analysis carried out within strata of alcohol consumption, there was no suggestion of effect modification. Consistent with our results, three prospective cohort studies, conducted in Japan( Reference Hara, Sasazuki and Inoue 26 ), the USA( Reference Zhang, Giovannucci and Smith-Warner 13 ) and Canada( Reference Kabat, Miller and Jain 14 ), showed that the association of the intake of total Fe and haem Fe with colorectal cancer risk did not differ by the amount of alcohol consumed. However, two female cohort studies in Iowa( Reference Lee, Anderson and Harnack 24 ) and Sweden( Reference Larsson, Adami and Giovannucci 25 ) reported that the association of colon cancer with haem Fe intake was particularly strong among women who consumed at least 10 g of alcohol/d( Reference Lee, Anderson and Harnack 24 ) or 20 g of alcohol/week( Reference Larsson, Adami and Giovannucci 25 ). The stronger associations observed among alcohol drinkers in these two studies may be due to chance because of the relatively small number of cases in the highest quintile of haem Fe and alcohol consumption categories (eight cases( Reference Lee, Anderson and Harnack 24 ) and forty-five cases( Reference Larsson, Adami and Giovannucci 25 ), respectively). Further studies with larger sample size are needed to investigate the interaction with alcohol consumption.
Stratified analysis by sex showed that no significant association was found between total dietary Fe, non-haem Fe and colorectal cancer risk in both sexes, whereas the inverse association of Fe from plants and the positive associations of haem Fe, Fe from meat and Fe from red meat and colorectal cancer risk were only observed among males but not females. These sex differences have been explored in some previous studies, however, the results were controversial( Reference Zhang, Giovannucci and Smith-Warner 13 , Reference Balder, Vogel and Jansen 16 , Reference Deneo-Pellegrini, De Stefani and Boffetta 20 , Reference Wurzelmann, Silver and Schreinemachers 21 , Reference Hara, Sasazuki and Inoue 26 ). There are several plausible explanations for the different findings. First, it was reported that males are known to consume more food in general and more meat and relatively less vegetable compared with females( Reference Northstone 61 ). Different combinations of food groups or nutrients in the diets of males and females may have distinct effects on the carcinogenesis of colorectal cancer. Moreover, the recommended nutrient intake of total dietary Fe intake was 15 mg/d for males and 20 mg/d for females in China( Reference Yang, Wang and Pan 38 ). Relatively more haem Fe is absorbed in females due to menstrual losses( Reference Hunt 62 ), so that higher haem Fe intake is more likely to form the cytotoxic factor in the bowel and further leads to neoplasm among males.
Subgroup analysis by cancer site presented that intake of haem Fe and Fe from meat were only observed to be positively associated with colon cancer risk. In agreement with our result, a meta-analysis consisting of five prospective studies suggested that higher haem Fe intake was associated with increased risk of colon cancer( Reference Bastide, Pierre and Corpet 23 ). The Iowa Women’s Health Study found a positive relationship between haem Fe intake and proximal colon cancer( Reference Lee, Anderson and Harnack 24 ). Meanwhile, some studies detected null association between haem Fe and rectal cancer( Reference Zhang, Giovannucci and Smith-Warner 13 , Reference Lee, Anderson and Harnack 24 , Reference Hara, Sasazuki and Inoue 26 ). It has been reported that the concentration of intraluminal Fe was higher in the proximal tract( Reference Lund, Wharf and Fairweather-Tait 63 ), which to some extent might explain the apparent pernicious effect found in colon cancer. Furthermore, colon and rectal cancers have different clinical features and genetic characteristics. The different pH levels( Reference Wei, Giovannucci and Wu 64 ) and microbiota composition of the cancer site( Reference Macfarlane and Macfarlane 65 ) may affect their susceptibility to diet components( Reference Koushik, Hunter and Spiegelman 66 ). Nevertheless, further exploration is needed to confirm the relationship.
Our study has the following strengths. First, this is the first study to examine the association between different forms and sources of Fe and colorectal cancer risk in the Chinese population. Second, information was collected on a wide range of potential confounders including dietary and non-dietary factors and adjusted in the analysis. Third, the sample size in the present study was larger than that in all previously published case–control studies. We had adequate power to explore the associations between Fe intake and colorectal cancer risk.
The present study had some limitations. First, selection bias is difficult to rule out in hospital-based case–control studies. In the present study, the colorectal cancer patients were consecutively recruited from Sun-Yat-sen University Cancer Center, which is the biggest cancer centre in Southern China. The colorectal cancer patients at this centre shared similar clinical characteristics as those from other big hospitals in Guangdong or in mainland China( Reference Xu and Jiang 67 , Reference Li, Huang and Shi 68 ). Moreover, the high participation rate (89 % for cases and 87 % for hospital-derived controls) also helped to reduce selection bias in our results. Second, recall bias is also a concern in case–control studies. To diminish this bias, we made great efforts to interview the cases as soon as diagnosis was made. The average time interval between the diagnosis of colorectal cancer and study interview was 10·3 d for the case subjects. Photographs of foods with the usual portion size were also provided to help study subjects accurately estimate the food intake. Finally, in the present study, Fe from dietary supplements was not calculated as part of the exposure. This might limit the evaluation of association between total Fe intake and colorectal cancer risk. However, it was reported that in China only 1·3 % of adults took Fe supplements( Reference Ma, Cui and Li 69 ). Therefore, the potential influence of Fe dietary supplements should not be a serious problem.
In conclusion, this study showed that lower intake of Fe from plants and white meat, as well as higher intakes of haem Fe and Fe from red meat, were associated with the risk of colorectal cancer in a Chinese population. However, intake of total dietary Fe, non-haem Fe and Fe from meat were not associated with colorectal cancer risk. The associations between Fe intake and colorectal cancer risk were not modified by alcohol consumption. In particular, the results of this study highlighted the importance of different forms and sources of Fe intake, which might exert different impacts on colorectal cancer prevention. These results may provide some recommendations for the prevention of colorectal cancer, such as reducing the consumption of Fe from red meat and increasing the consumption of Fe from plants or white meat.
Acknowledgements
The authors gratefully acknowledge the contribution of the study participants, without whom the study would not have been possible.
This study was supported by Guangdong Natural Science Foundation (no. 2016A030313225, 2014A030313188). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities are as follows: H. L. conducted the data collection and analysed the data and writing of this paper. N.-Q. Z., J. H., X. Z. and X.-L. F. participated in the data collection and data entry. Y.-J. F. and Z.-Z. P. were responsible for connecting and coordinating the field work. Y.-J. F. and Y.-M. C. provided significant advice regarding the analyses and interpretation of the data. C.-X. Z. constructed the project design and supervised and contributed to the manuscript writing.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000023











