It is clear that the intestinal microbiota provides the first line of defence against pathogenic organisms( Reference Falk, Hooper and Midtvedt 1 , Reference Stecher and Hardt 2 ). However, it is becoming more apparent that it also exerts a major influence over host homeostasis in healthy humans and animals( Reference Lewis, Inman and Patel 3 – Reference Schmidt, Mulder and Musk 6 ). The microbiota can be altered by factors such as diet( Reference Noverr and Huffnagle 7 ) and environment( Reference Mulder, Schmidt and Stokes 4 – Reference Schmidt, Mulder and Musk 6 ), but in adults, the mature microbiota tends to re-establish itself once the external influence is removed( Reference Moore and Moore 8 ). More long-term alterations may be generated during early life, when this intestinal ecosystem is still fluctuating( Reference Palmer, Bik and DiGiulio 9 ) and highly susceptible to change( Reference West, Hammarstrom and Hernell 10 , Reference Cox, Huang and Fujimura 11 ). The process of microbial colonisation and succession in the intestine is a major factor in driving maturation of the immune system( Reference Carter and Pollard 12 – Reference Butler, Weber and Sinkora 16 ), and the composition of the microbiota can affect the function of the immune system in neonates and adults( Reference Ivanov, Frutos Rde and Manel 17 ). Some specific modifications of the microbiota have been correlated with disease( Reference West, Hammarstrom and Hernell 10 , Reference Bjorksten, Sepp and Julge 18 , Reference Sekirov, Tam and Jogova 19 ), and clinical trials have suggested that strain-specific probiotic therapy can confer a health benefit for specific disease situations( Reference Iannitti and Palmieri 20 – Reference Zakostelska, Kverka and Klimesova 24 ). The process of weaning is associated with a major shift in the gut microbial community in both humans( Reference Magne, Hachelaf and Suau 25 ) and other mammals( Reference Konstantinov, Awati and Williams 26 ), and therefore may present a target for beneficial manipulation of the microbiota. Intervention with probiotics during weaning may have a more pronounced impact on the subsequent function of the immune system than administration of probiotics to adults.
Assessing the likely value of a probiotic in a specific clinical situation requires either direct measurement of health benefit as part of a clinical trial, or an understanding of the mechanism of action of the probiotic. The size and robustness of the evidence base will allow rational selection of specific probiotic strains and the clinical situations in which trials are likely to have positive outcomes. However, the extent to which mechanistic studies can be carried out in human subjects is limited. Neonates of altricial species such as rodents are not easily manipulated: in contrast, the omnivorous pig is not only similar to humans in terms of anatomical, physiological, immune and metabolic characteristics( Reference Gandarillas and Bas 27 – Reference Tadros, Traber and Heggers 31 ) but, in addition, their precocial development makes them appropriate candidates for manipulation around weaning.
Effects of early-life environment on microbial colonisation have been identified in young piglets( Reference Mulder, Schmidt and Stokes 4 ), and intra-individual stability and inter-individual variability of the microbiota are more similar between humans and pigs than between humans and mice( Reference Thompson, Hofer and Campbell 32 ). Further, full genome studies have demonstrated less differences between humans and pigs than between humans and rodents( Reference Wernersson, Schierup and Jorgensen 33 , Reference Jorgensen, Hobolth and Hornshoj 34 ). These factors suggest that piglets are a valuable intermediate between highly reductionist, mechanistic studies in mice, and human epidemiological studies and clinical trials, especially with regard to weaning and nutritional intervention. Here we use a healthy piglet weaning model to identify the effects of intervention with the human probiotic Bifidobacterium lactis at weaning on immunological development and function, and question how well generally accepted proxy measures of health truly reflect the physiological status of an individual.
Materials and methods
Animals
Animal housing and experimental procedures were all performed according to local ethical guidelines: all experiments were performed under a UK Home Office License and were approved by the local ethical review group. A total of seven outbred sows were artificially inseminated using semen from a single boar (supplied by Hermitage-Seaborough Limited). Sows were transported to the Department of Clinical Veterinary Science 6 weeks before parturition and fed on a wheat-based diet (BOCM Pauls Limited). At 3 weeks of age, piglets were weaned and litter-matched into six groups (Table 1), each group being housed in a separate room, on straw, in standard large animal facilities.
Table 1 Experimental design*

i.p., Intraperitoneally; OVA, ovalbumin.
* Forty-two (six piglets from seven litters) piglets were litter-matched into six treatment groups; three groups received the B. lactis NCC2818 intervention at weaning at 3 weeks of age.
At this point, three groups received the B. animalis subsp. lactis (CNCM I-3446) probiotic diet supplementation in the form of spray-dried culture mixed into the formula at a concentration of 4·2 × 106 colony-forming units/ml (approximately 2 × 109 colony-forming units/kg metabolic weight per d). The required quantity of feed supplemented with fresh probiotics was fed twice per d to the appropriate groups (A–C) until the experiment concluded when the pigs were 11 weeks old. The remaining groups (D–E) did not receive the probiotic supplement. The probiotic-fed and control animals were in different suites separated by a biosecurity barrier. Of the piglets receiving probiotics, two groups were weaned onto a soya-based diet and one onto an egg-based diet (Table 2). All diets were supplemented with appropriate levels of vitamins and minerals and were manufactured to order by Volac (Parnutt) Foods Limited. The weaning diets were designed such that the only differences were that each contained 21 % of the stated protein. One of the two groups that weaned onto the soya-based diet also received an intraperitoneal injection of 2 mg soluble ovalbumin from chicken egg-white (systemic exposure; Sigma) and 2 mg Quil A adjuvant (Brenntag Biosector A/S) in 2 ml PBS to investigate the immune response against a systemically administered novel protein. The treatment of these three probiotic-supplemented groups (A–C) was replicated in the probiotic-free control groups D to F. From 7 weeks of age, all six groups were fed a fishmeal-based diet, free of egg and soya, either with or without probiotic as appropriate. The fishmeal diet was used to ensure that the serum antibody response was to the injected egg protein and not to the dietary egg protein. The egg- and soya-based diets were designed to meet the nutritional requirements of piglets between 3 and 7 weeks old, whereas the fishmeal-based diet was designed for piglets between 7 and 11 weeks old. For this reason, the fishmeal-based diet cannot be compared with the egg- and soya-based diets. The composition of the different diets is shown in Table 2. At 9 weeks old, all piglets received an intraperitoneal injection of 2 mg ovalbumin and 2 mg Quil A adjuvant in 2 ml PBS.
Table 2 Composition of the weaning diets and supplements
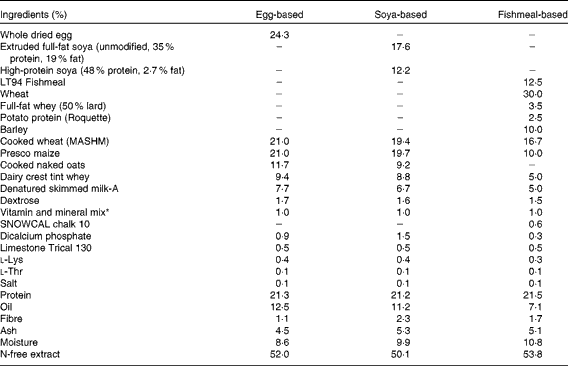
* Vitamin and mineral mix (calculated units in finished feed): vitamin A, 16 mg/kg; vitamin D3, 2 mg/kg; vitamin E, 250 mg/kg; vitamin K (menadione), 4 mg/kg; vitamin B1, 10 mg/kg; vitamin B2, 16 mg/kg; vitamin B6, 10 mg/kg; vitamin B12, 0·05 mg/kg; nicotinic acid, 50 mg/kg; pantothenic acid, 30 mg/kg; biotin (vitamin K), 0·2 mg/kg; vitamin C, 200 mg/kg; folic acid, 3 mg/kg; choline chloride, 300 mg/kg. Trace minerals: Cu, 155 mg/kg; Fe, 375 mg/kg; Zn, 110 mg/kg, Mn, 100 mg/kg; Co, 0·5 mg/kg; I, 1·2 mg/kg; Se, 0·3 mg/kg.
All piglets were bled by venepuncture at 3, 4, 5, 7 and 9 weeks old for collection of serum. At 11 weeks old, piglets were sedated with azaperone and killed with an overdose of barbiturate. At post-mortem, heart blood and tissues were recovered.
Tissue culture
At killing, 4 cm2 samples of intestinal mucosa (proximal and distal jejunum, excluding Peyer's patches (PP), distinct jejunal PP, caecum and descending colon), and 1 cm3 of spleen and mesenteric lymph node (MLN) were collected and placed in cold sterile medium. Organ fragment culture was carried out as described in detail by Logan et al. ( Reference Logan, Chow and George 35 ). Briefly, the samples were vigorously washed three times in Ca2+ and Mg2+-free Dulbecco's PBS (Sigma) containing 0·5 mm-EDTA (Sigma), 1 m-HEPES (Invitrogen) and 50 μg gentamycin/ml (Gibco), followed by three further washes in Ca2+ and Mg2+-free Dulbecco's PBS containing 1 % HEPES and 50 μg gentamycin/ml before being placed in Roswell Park Memorial Institute-1640 medium (Sigma) containing 10 % fetal calf serum (PAA), 200 mm-l-glutamine (Invitrogen), 10 units penicillin/ml and 10 μg streptomycin/ml (Invitrogen) and 50 μg gentamycin/ml (complete medium). Intestinal tissues were cut into fragments approximately 3 mm2, while spleen and MLN were cut into 2 mm cubes, and one fragment of tissue was placed in each of six individual wells of a twenty-four-well culture plate (Corning, Inc.) containing 1 ml of complete medium. Cultures were incubated at 37°C, 5 % CO2, 100 % humidity for 96 h, after which they were frozen at − 20°C. The plates were defrosted and the spent medium from each of the six duplicate wells for each sample was pooled and refrozen for analysis of Ig content.
Immunoglobulin assays
Catching ELISA was carried out to determine total IgG1, IgG2, IgA and IgM in spent medium from organ fragment cultures and IgA in serum. Briefly, ninety-six well microplates were coated with either affinity-purified goat anti-pig IgG (H+L), goat anti-pig IgA or goat anti-pig IgM (Bethyl Laboratories). Serial dilutions of serum samples and reference standard were added to coated plates and incubated for 2 h at room temperature. Bound Ig were detected using isotype-specific monoclonal antibodies (anti-pig IgA K61.1B4, anti-pig IgM K52.1C3, anti-pig IgG1 K139.3C8 and anti-pig IgG2 K68.1G2; all from Serotec) followed by horseradish peroxidase-conjugated goat anti-mouse IgG1. Concentrations of Ig subclasses were determined by interpolation of samples onto the reference standards.
Antigen-specific immunoglobulin assays
Serum samples were analysed for anti-ovalbumin IgG1 and IgG2 antibodies by ELISA as described in detail by Bailey et al. ( Reference Bailey, Haverson and Miller 36 ). Briefly, ninety-six-well microplates were coated with ovalbumin from chicken egg-white (Sigma) before non-specific binding sites were blocked with 2 % bovine serum albumin (Sigma) in PBS–Tween 20. After washing, serial dilutions of serum samples and reference standard were added to the plates. Reference standard was porcine serum obtained following hyperimmunisation with ovalbumin. Bound anti-soya IgG1 and IgG2 antibodies were detected using isotype-specific monoclonal antibodies followed by HRP-conjugated goat anti-mouse as mentioned previously, and relative concentrations of antibody were determined by interpolation of samples onto the reference standards.
In order to compare changes in serum antibody generated by weaning and by the injection of novel proteins in outbred animals, in which the starting levels differ, results are expressed as the ratio of antibody after manipulation to that before manipulation (fold change in antibody).
Immunohistology
Sample collection
MLN and caecum tissue was removed shortly after death from each of the experimental piglets. Tissues were embedded in OCT (Tissue TEK; BDH), snap-frozen in isopentane and pre-cooled to approximately − 70°C in the vapour phase of liquid N2. Samples were stored at − 80°C until sectioning. Serial 5 μm sections of these tissues were cut using a Model OTF cryotome (Bright Instrument Company Limited). Sections were air-dried for 24 h and then fixed by immersion in acetone for 15 min. Slides were allowed to dry before storage at − 80°C.
Fluorescence immunohistology
For two-colour fluorescence immunohistology, mouse anti-pig monoclonal antibodies (IgA and IgM, as for ELISA) were used to identify free and cell-bound IgA- and IgM-positive cells and B-lymphocytes (anti-CD21, clone IAH CC55). The conjugated secondary reagents used were as follows: goat anti-mouse IgG1 conjugated to fluorescein isothiocyanate (FITC) (Southern Biotechnology, AMS Biotechnology) and goat anti-mouse IgG2b conjugated to tetramethyl rhodamine isothiocyanate (TRITC) (Southern Biotechnology). Tissue staining, image capture and automated image analyses were carried out as described by Inman et al. ( Reference Inman, Rees and Barker 37 ) with the exception that fracture crystallography (Fc) receptor blocking was achieved using 10 % goat serum in PBS.
Histochemistry
Small-intestinal samples were obtained as described in the immunohistology section and processed the same up to and including the acetone fixation step. Fixed slides were stained for mast cells using 2·5 % toluidine blue O solution (Sigma-Aldrich) for 15 s followed by dehydration through increasing concentrations of alcohol culminating in a histoclear® (National Diagnostics) wash and mounted in distyrene plasticiser xytene (DPX) mounting medium (Fisher). Image capture was carried out using a Colour Coolview camera and ImagePro Plus software (Photonic Science). Thereafter, ten fields of view were obtained from each piglet and ImageJ software (National Institutes of Health) was used to allow quantification of mast cells per cm2 tissue.
Statistical analysis
Statistical analysis was carried out using SPSS statistics (SPSS, Inc.). Univariate linear regression was carried out using piglet as the experimental unit and litter, tissue and probiotic treatment as variables. Individual differences between the treatment groups were determined by least significant differences as in our previous experiments( Reference Lewis, Inman and Patel 3 ).
Results
Local immunoglobulins
Bifidobacterium lactis NCC2818 supplementation caused a reduction in local immunoglobulin production in lymphoid-associated organ fragment cultures
Total IgG1, IgG2, IgA and IgM were quantified in organ fragment culture medium from all animals. There were highly significant differences in the amounts of the four isotypes produced between tissues (P< 0·0001), spleen producing less IgA ( − 0·16 (sem 0·04) log10 μg/ml) than mucosal tissues in the control animals (mean range − 0·5–1·16 log10 μg/ml). Highly significant effects of probiotic intervention were observed for IgA (P< 0·0005; Fig. 1(a) and (b)) and IgM (P< 0·009; Fig. 1(c) and (d)), but not for IgG1 or IgG2 (data not shown). IgA and IgM were lower in the probiotic supplemented animals than in the unsupplemented animals (for IgA from MLN, − 0·56 (sem 0·09) and 0·34 (sem 0·05) log10 μg/ml, respectively; from proximal jejunum, 0·75 (sem 0·03) and 0·86 (sem 0·01) log10 μg/ml; from jejunal PP, 0·02 (sem 0·09) and 0·80 (sem 0·09) log10 μg/ml; from caecum, 0·74 (sem 0·06) and 1·17 (sem 0·05) log10 μg/ml; for IgM from MLN, 0·38 (sem 0·08) and 0·72 (sem 0·03) log10 μg/ml, respectively; from caecum, 0·13 (sem 0·18) and 0·85 (sem 0·03) log10 μg/ml; from colon, 0·60 (sem 0·04) and 0·81 (sem 0·03)) log10 μg/ml). There was also a significant interaction between probiotic treatment and tissue (P< 0·0001 for both classes), such that this effect was more marked for some tissues than others. Specifically, probiotic intervention appeared to have the most marked effect on IgA production by the organised tissues of MLN and jejunal PP (Fig. 1(a)), and to a lesser extent by the diffuse lymphoid tissue present in caecal mucosa (Fig. 1(b)). Although supplementation also resulted in significantly decreased IgA production by tissue from the proximal small intestine, the effect was much smaller. IgA production in the spleen, distal small intestine and colon showed no difference between the probiotic supplemented and non-supplemented animals (from spleen, − 0·25 (sem 0·04) and − 0·16 (sem 0·04) log10 μg/ml, respectively; from distal jejunum, 0·46 (sem 0·03) and 0·44 (sem 0·07) log10 μg/ml; from colon, 1·16 (sem 0·02) and 1·06 (sem 0·05) log10 μg/ml). There was also no difference as a result of dietary supplementation in spleen or small-intestinal IgM (for spleen, 0·62 (sem 0·09) and − 0·79 (sem 0·06) log10 μg/ml, respectively; from proximal jejunum, 0·36 (sem 0·07) and 0·36 (sem 0·07) log10 μg/ml; for distal jejunum, 0·27 (sem 0·06) and 0·40 (sem 0·05) log10 μg/ml; for discrete jejunal PP, 0·75 (sem 0·05) and 0·35 (sem 0·05) log10 μg/ml). It should be noted that IgA in serum taken at time points throughout the experiment remained unaltered by the supplementation with B. lactis NCC2818 (P>0·05).

Fig. 1 Total (a, b) IgA and (c, d) IgM production (μg/ml, log-transformed) by organ fragment cultures from organised (a, c) lymphoid tissues and (b, d) non-lymphoid tissues from piglets supplemented with Bifidobacterium lactis NCC2818 (groups A, B and C) or unsupplemented (groups D, E and F). Values are means, with their standard errors represented by vertical bars (n 21). a,bMean value with unlike letters was significantly different from that of probiotic supplementation (P< 0·01; t test with Bonferroni correction). MLN, mesenteric lymph node; JPP, jejunal Peyer's patches; Pro SI, proximal small intestine; Dis SI, distal small intestine. ■, B. lactis; □, control.
Local IgA and IgM proteins were reduced in caecal tissue, mesenteric lymph node-associated B-cells and B-cell follicles following probiotic intervention
In order to examine the mechanisms by which probiotic administration reduced Ig secretion in organ fragment cultures, levels of IgA, IgM and CD21 were examined in MLN (Figs. 2 and 3) and caecum (Fig. 4) samples from groups B and E (soya diet, supplemented with the probiotic and control, respectively). These tissues and groups were chosen as they had previously produced the most consistent differences in organ fragment cultures. Consistent with the organ fragment culture data, there was reduced expression of IgA (Fig. 2(a) and (d)), IgM (Fig. 2(d)) and also CD21 (Fig. 2(c)), within B-cell follicles in the MLN of the B. lactis NCC2818-treated animals compared with the control group (P< 0·0001). There was no effect of intervention with B. lactis NCC2818 on the total number of MLN follicles (P>0·05; Fig. 3(a)), but the number of IgM-specific follicles (Fig. 3(c)) and extrafollicular IgM-producing B-cells (Fig. 3(b)) was reduced in the animals receiving the intervention (P< 0·0001). In contrast, no change was seen in the number of extrafollicular IgA-positive cells (Fig. 3(b), P>0·05), whereas the number of IgA-specific follicles was actually significantly increased (Fig. 3(c)) in animals fed with B. lactis NCC2818 when compared with the controls (P< 0·0001). Reductions in the expression of IgA (Fig. 4(a) and (c)) and IgM (Fig. 4(b) and (d)) in situ in the caecum were also apparent (Fig. 4), both in the subepithelial lamina propria (associated with production) and in the caecal crypt epithelium (associated with transport) (Fig. 4(c) and (d)).

Fig. 2 Fluorescence immunohistology of the mesenteric lymph node from treatment groups B (ovalbumin priming and recall and Bifidobacterium lactis NCC2818 intervention; ■) and E (ovalbumin priming and recall without B. lactis NCC2818 intervention; □). (a) Example field from treatment group B: green fluorescence indicates binding of anti-pig IgA monoclonal antibody and red, anti-pig CD21 monoclonal antibody. (b) Example field from treatment group E stained similarly. (c) Proportional area of expression of CD21 and (d) IgA and IgM in the same treatment groups (group B, B. lactis NCC2818; group E, control). Values are means, with their standard errors represented by vertical bars (n 7). *** Mean value was significantly different from that of group B (P< 0·0001 in all cases). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)

Fig. 3 B-cell follicles in the mesenteric lymph node from soya-fed piglets that received intraperitoneal ovalbumin priming and recall and Bifidobacterium lactis NCC2818 intervention (treatment group B; ■) or no B. lactis NCC2818 intervention (treatment group E; □), identified by fluorescence immunohistology. (a) Total number of follicles present in tissues: no significant difference between the animals receiving the B. lactis NCC2818 intervention and those that did not. (b) Extrafollicular cells containing either IgA or IgM. (c) Number of IgA- or IgM-positive follicles. Values are means, with their standard errors represented by vertical bars (n 7). *** Mean value was significantly different from that of the control group (P< 0·0001).

Fig. 4 Fluorescence immunohistology of caecum crypts and lamina propria from soya-fed piglets which received intraperitoneal ovalbumin priming and recall (treatment groups B and E) and either (a) Bifidobacterium lactis NCC2818 supplementation or (b) not; fluorescence indicates the binding of anti-pig IgA monoclonal antibody. (c) IgA and (d) IgM fluorescence quantified (P< 0·0001 in all cases). Values are means, with their standard errors represented by vertical bars (n 7). ■, B. lactis; □, control. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
A reduction in lymphoid-associated IgA and IgM production was associated with increased mast cell numbers in the intestinal mucosa
In contrast to the decreases observed in IgA and IgM in the supplemented animals, there were significantly greater numbers of mast cells in the small intestine (P< 0·001) of animals which received B. lactis NCC2818 (n 7) when compared with the control (Fig. 5).

Fig. 5 Mast cell counts in the lamina propria of the proximal small intestine from piglets (n 7) weaned onto a soya diet and given intraperitoneal ovalbumin priming and recall (treatment groups B and E). Mast cells identified by toluidine blue staining. Symbols indicate litter-matched animals (P< 0·0001).
Systemic antibody
Primary systemic response to novel fed protein at weaning was increased following Bifidobacterium lactis NCC2818 administration
At weaning, there was a significant increase in IgG2 anti-soya antibody in animals which received a soya diet with probiotic supplementation when compared with both the animals fed soya without probiotic and the egg-fed animals (P= 0·021; Fig. 6(a)). The supplemented animals also mounted a significantly greater IgG1 antibody response to soya compared with the egg-fed controls (P= 0·03), and a greater response than the unsupplemented animals fed soya, although this was not significant (Fig. 6(b)).

Fig. 6 Increased serum soya-specific (a) IgG2 and (b) IgG1 antibody in response to weaning onto a soya-based diet in piglets receiving Bifidobacterium lactis NCC2818 supplementation (groups B and C) compared with the non-supplemented groups (groups E and F). Values are means, with their standard errors represented by vertical bars (n 14). IgG1 anti-soya increase was not significant on its own, but combined analysis of changes with anti-soya IgG2 increased the P value to P= 0·05. For IgG2, P= 0·03. ![]() , Soya+B. lactis;
, Soya+B. lactis; ![]() , soya diet;
, soya diet; ![]() , egg diet.
, egg diet.
Primary and secondary responses to injected antigens were increased following probiotic intervention at weaning
Fig. 7(a) and (b) shows the increase in serum IgG1 and IgG2 antibody, respectively, during the primary and secondary responses to systemically injected antigens (with the adjuvant). During the primary response (3–5 weeks old), there were trends towards an increased serum IgG1 and IgG2 anti-ovalbumin response in the B. lactis NCC2818-fed animals (n 7) compared with the control group. During the secondary response (9–11 weeks old), there was a significantly greater response in both isotypes in the supplemented group (IgG1, P= 0·05 and IgG2, P= 0·02).

Fig. 7 Increased piglet serum ovalbumin-specific (a) IgG1 and (b) IgG2 antibody in response to priming and recall with intraperitoneal ovalbumin with the Quil A adjuvant in piglets weaned onto the soya diet, with and without the Bifidobacterium lactis NCC2818 intervention. Results for antibodies are given as a proportion of the standard. Values are means, with their standard errors represented by vertical bars (n 7). * P values for IgG1 and IgG2 are 0·05 and 0·02, respectively. Combined analysis of changes gives a P value of 0·004. ■, B. lactis; □, control.
Discussion
The common definition of a probiotic (given by the WHO in 2001) is ‘a live microorganism that when administered in adequate amounts confers a health benefit on the host’. Thus, the definitive outcome measure necessary when testing novel strains for probiotic activity is health: in normal or diseased humans or animals, this may be measured, for example, by susceptibility to disease. However, understanding the mechanisms by which probiotics function requires detailed measurement of a wider range of immunological and physiological parameters, which may then also be used as proxy measures of health. The strain of B. lactis NCC2818 used in the present experiments has been identified as having probiotic activity, as defined above, in human subjects and in rodent models. These benefits include reducing pathogen load and prevention or reduction of antibiotic-associated diarrhoea( Reference Ishibashi and Shimamura 38 , Reference Harata, He and Takahashi 39 ). However, the effects of probiotics, including B. lactis NCC2818, on immune development at weaning, a time when the resident microbiota is changing rapidly, are largely unknown. The immunological measures reported here, then, relate to the mechanisms of action of the probiotic strain and to the identification of proxy measures of the probiotic effect. The results presented clearly demonstrate that administration of B. lactis to piglets at weaning had marked effects on the structure and function of the mucosal immune system. In that respect, the present results are comparable with mechanistic experiments in rodents and with the data from human clinical trials.
In our system, intervention with B. lactis NCC2818 resulted in reduced IgA in mucosal-associated lymphoid tissues (associated with a reduction in plasma cell numbers by immunohistology). In contrast, preterm infants, which received B. lactis NCC2818 for 3 weeks following birth, showed a 2-fold increase in faecal IgA levels from 2 weeks onwards( Reference Mohan, Koebnick and Schildt 40 ), and IgA production by MLN and PP cells from adult mice was increased when cultured in the presence of Bifidobacterium bifidum ( Reference Park, Um and Lee 41 ). In a mouse study, IgA in the intestinal fluids of the supplemented animals was also higher than that in the controls. In these and previous studies, elevated secretory IgA has been presumed to be a mechanism or a proxy measure for a beneficial effect of probiotics( Reference Kukkonen, Kuitunen and Haahtela 42 ), presumably by increasing the potential for neutralisation of allergens or pathogen, thus preventing or reducing disease. The disparity between the present results and those reported in human subjects and rodents may be more apparent than real. Where intervention has resulted in increased IgA levels in vivo, it should be noted that faecal IgA levels and total intestinal washes, as normally carried out in these species, may be more reflective of jejunal and/or colonic mucosal IgA levels, where there was no effect of supplementation in pigs of the present study, rather than the MLN, PP and caecum, where there was an effect. In addition, studies in human subjects have frequently involved compromised individuals( Reference Mohan, Koebnick and Schildt 40 ), whereas the present study used normal, outbred healthy piglets. While an increase in faecal IgA has been correlated with protection, the same correlation has not been established for local tissue IgA. Interestingly, an increase in intestinal IgA can be linked to various disease states in humans( Reference Berstad, Kilian and Valnes 43 – Reference Valnes, Brandtzaeg and Elgjo 45 ), and a local increase of IgA in a healthy individual can also be an indication of the loss of barrier function. We thus suggest that the present observation that probiotic supplementation decreased, rather than increased, local IgA production in intestinal tissue reflects a reinforcement of the intestinal barrier (preventing exposure to luminal antigens) rather than a suppression of mucosal immunity, and a breakdown in barrier function is often associated with disease. It also suggests that while elevated IgA in the faeces is accepted as a proxy measure for health, the same interpretation cannot necessarily be applied to local IgA production in tissues.
Similarly, although elevated numbers of mast cells have been associated with allergic sensitisation( Reference Sun, Li and Li 46 ), the increases in mast cell numbers seen here were within the normal ranges previously reported in young piglets( Reference Smith, Clark and Overman 47 , Reference Che, Pang and Hua 48 ), and are in line with physiological numbers in adult pigs( Reference Duncker, Lorentz and Schroeder 49 ) and were not comparable with those seen in disease states( Reference Gelbmann, Mestermann and Gross 50 , Reference Crowe, Luthra and Perdue 51 ). An increase in mast cell numbers within the normal physiological range may be a consequence of increased recruitment to the intestinal mucosa, decreased mast cell exit or the inhibition of mast cell degranulation. Previous studies have suggested that mast cell degranulation contributes to impaired barrier function after weaning in young piglets, and several probiotic species have been shown to reduce IgE-mediated degranulation in an RBL-2H3 cell line( Reference Harata, He and Takahashi 39 , Reference Crowe, Luthra and Perdue 51 ). A reduction in antigen-induced mast cell degranulation may also occur as a consequence of elevated IgG antibody responses to fed and injected antigens in probiotic supplemented animals: elevated serum IgG antibody responses to food proteins have been associated with decreased susceptibility to IgE-mediated allergic disease in human subjects and to post-weaning diarrhoea in pigs( Reference Li, Nelssen and Reddy 52 , Reference Strait, Mahler and Hogan 53 ). Further, since active, primary responses to intestinal antigens are largely mediated through PP( Reference Snoeck, Verfaillie and Verdonck 54 ) while tolerance is mediated by the transfer of antigens from the intestinal mucosa to the MLN( Reference Hadis, Wahl and Schulz 55 ), stronger responses to fed antigens in supplemented piglets may also indicate reduced uptake across the intestinal epithelium compared with PP. The present results strongly suggest caution in interpreting specific measures of the immune system (in this case, IgA production, mast cell numbers and antibody to food proteins) as linear, proxy measures for the health benefit of probiotic supplementation in the diet without taking the specific animal model and, more importantly, the specific intervention window into account.
Mechanistically, the present results are largely consistent with B. lactis NCC2818 intervention, increasing barrier function between the lumen and the intestinal lamina propria. Specifically, a reduction in IgA production in organ fragment cultures is entirely consistent with a reduced exposure of the mucosal immune system to antigens derived from the intestinal lumen. Previously, certain probiotics, including bifidobacteria, have been shown to enhance the barrier function of human intestinal epithelial cells in vitro ( Reference Resta-Lenert and Barrett 56 ), but not in vivo, in part by the stabilisation of tight cell junctions( Reference Otte and Podolsky 57 ). B. bifidum, for example, was demonstrated to increase barrier integrity in a rat model of neonatal necrotising enterocolitis( Reference Khailova, Dvorak and Arganbright 58 ).
In conclusion, the present results demonstrate clear effects of probiotic supplementation in the weaning diets of conventionally reared animals which do not have any diseases or unusual pathology. Mechanistically, these effects are consistent with increased barrier function. However, the results also strongly suggest that while measures of the effect of probiotic supplementation on the immune system are of value in developing an understanding of the mechanism of action, we may need to interpret with caution. While studies of health benefits are appropriately conducted in human subjects, mechanistic studies require tractable animal models from which sufficient tissue samples can be easily recovered. Such mechanistic studies should, perhaps, be carried out in several mammalian species in order to establish generally applicable principles for predicting the activities of probiotic strains.
Acknowledgements
The present study was supported by Nestec Limited, which also supplied the probiotic B. lactis NCC2818. We wish to thank Stuart Ham for his assistance with the pigs. The authors' contributions are as follows: M. C. L., M. B., A. W. Z., A. M. and S. D. developed the overall research plan; M. C. L., D. V. P., M. B. and J. F. conducted the research; M. C. L. and M. B. analysed the data, performed the statistical analysis and wrote the paper; M. C. L. had primary responsibility for the overall direction and final content. All authors read and approved the final manuscript. M. C. L., M. B., D. V. P. and J. F. declare no conflict of interest. A. W. Z., A. M. and S. D. are employees of Nestec Limited.













