Stimulation of selected cells related to the immune system by immune stimulants triggers systemic metabolic changes including fever, anorexia, skeletal muscle protein deposition, and consequently affects growth. IL-1β and TNF-α are major pro-inflammatory cytokines and are regulators of host responses to infection, immune responses, inflammation and trauma(Reference Dinarello1–Reference Humphrey and Klasing3). Glycine (Gly) is a non-essential amino acid in mammals. There is experimental evidence that Gly has a role in protecting tissue from damage and improves survival rate in endotoxaemic and ischaemic mice and rats through modulation of TNF-α, IL-1β and IL-10 secretion(Reference Grimble, Jackson, Persaud, Wride, Deleres and Engler4–Reference You, Wang, Li, Chen, Liu and Gong13), although Chambon-Savanobitch et al. (Reference Chambon-Savanobitch, Felgines, Farges, Raul, Cezard, Dabot, Vasson and Cynober14) observed that dietary supplementation with Gly did not bring about dramatic changes in metabolic responses in endotoxaemic rats. These results suggest that traumatic and inflammatory states in mammals may produce specific metabolic changes in which Gly is an essential nutrient.
Birds, including chickens, are able to biosynthesise Gly; however, their usual rate is insufficient to meet the bird's metabolic demand in the fast growing stage. In chicks, excess Gly can be converted to serine (Ser) on an equimolar basis and this reaction is reversible(Reference Sugahara and Kandazu15, Reference Klasing and Klasing16). Therefore, this dietary requirement is expressed as the ‘Gly+Ser’ level in standard poultry feed. It is advantageous to use chicks for investigating the potential anti-inflammatory properties of dietary Gly on anti-inflammatory properties, because the effect of Gly on growth performance has been studied to a great extent. It is presumed that stimulation of innate immune-related cells such as macrophages triggers systemic metabolic changes including fever, anorexia, skeletal muscle protein deposition, and therefore, affects growth. These processes are probably mediated by pro-inflammatory cytokines in chicks as in mammals(Reference Koutsos, Klasing, Garnsworthy and Wiseman2, Reference Humphrey and Klasing3). However, molecular cloning of TNF-α in avian species has proven unsuccessful, although TNF-like activity has been detected in the supernatant fraction of chicken macrophages(Reference Rautenschlein, Subramanian and Sharma17). Recently, chicken TNF-like ligand (TL) 1A facilitated investigation of synergetic actions of pro-inflammatory cytokines during inflammation, since chicken TL1A in chickens possibly functions as an alternative to the TNF-α of mammals(Reference Takimoto, Takahashi, Sato and Akiba18). It is possible that inflammatory conditions increase the Gly demand of birds at a stage of life when they exhibit an inherent requirement for this nutrient. It is also presumed that supplementation of Gly to poultry diets is a possible way to prevent the catabolic changes induced by immunological stimulation. However, there is little information available whether dietary Gly supplementation can modulate inflammatory responses in chicks.
The present study was undertaken to determine whether dietary supplementation with Gly modulates inflammatory responses and growth performance during immune stimulation in broiler chicks. As inflammatory response parameters, plasma ceruloplasmin (Cer) and nitrite plus nitrate (NOx), and the expression of IL-1β, IL-6, interferon (IFN)-γ, inducible NO synthase (iNOS) and toll-like receptor (TLR) 4 in the liver and spleen were examined.
Materials and methods
Animals, diet, blood sampling and lipopolysaccharide treatment
Unvaccinated 1-d-old male broiler chicks (Ross 308 strain) obtained from a local hatchery were used in all experiments. Birds were housed in electrically heated battery brooders and fed on a commercial starter diet (220 g crude protein/kg and 12·55 MJ metabolisable energy/kg) ad libitum for 7 d. For all experiments, 7-d-old chicks were selected to closest mean body weight to ensure body-weight uniformity and were reared in stainless-steel wire cages with two birds per cage in a temperature (25°C) and light (24 h/d) controlled room in all experiments.
In experiment 1, the effect of graded supplementation of the basal diet with Gly on plasma acute-phase substances following lipopolysaccharide (LPS) injection was determined. Forty chicks (7 d of age) were divided into four groups of ten chicks (five cages of two birds in each dietary group) and were provided with one of four experimental diets for 14 d ad libitum. The experimental diets were prepared by supplementation with crystalline Gly (Wako Pure Chemical Industries, Ltd, Osaka, Japan) at 10, 20 or 40 g/kg added to a basal diet. The basal diet contained 9·1 g Gly and 194 g Gly+Ser/kg diet as the calculated content. The composition of the basal and Gly-supplemental diets is shown in Table 1. Diets were formulated using maize and soyabean meal as the primary ingredients so as to ensure that all essential nutrients met (or exceeded) recommended levels as specified in the Japanese Feeding Standards for Poultry(19). At age 21 d, chicks were intraperitoneally injected with Escherichia coli LPS (serotype 0127:B8) at 1·5 mg/kg body weight. LPS was dissolved in sterile saline at a concentration of 500 μg/ml. A blood sample was taken from the brachial vein with a heparinised syringe just before, and 9 and 24 h following LPS injection; the sample was then centrifuged at 2500 rpm for 15 min at 4°C. Plasma was stored at − 80°C until further analysed. Previously published experiments performed under similar conditions(Reference Takimoto, Takahashi, Sato and Akiba18, Reference Takahashi, Kawamata, Akiba, Iwata and Kasai20) show that plasma NOx and Cer concentrations after LPS injection were near maximum at 9 and 24 h, respectively. A blood sample from each bird before LPS injection was used as its own individual control to permit calculation of changes in parameters for blood acute-phase inflammatory substances after LPS injection. We have previously shown that repeated blood sampling from the brachial vein does not significantly affect plasma concentrations of Cer(Reference Takahashi, Kawamata, Akiba, Iwata and Kasai20) and NOx(Reference Takimoto, Takahashi, Sato and Akiba18).
Table 1 Composition of the experimental diets in experiments 1 and 3

* See Akiba & Matsumoto(Reference Akiba and Matsumoto43).
In experiment 2, the effect of dietary supplementation with Gly on growth performance during immunological stimulation and plasma acute-phase substances following the first LPS injection was investigated under the condition of using isonitrogenous diets. Thirty-six chicks (age 7 d) were divided into three groups of twelve chicks (six cages of two birds in each dietary group) and were given the basal diet ad libitum, or the diet supplemented with Gly or supplemented with crystalline l-glutamic acid (Glu; Wako Pure Chemical Industries, Ltd, Osaka, Japan) for 14 d. The Gly- and Glu-supplemented diets were formulated to be isonitrogenous by supplementation of Gly (10 g/kg) plus cellulose (9·6 g/kg) or Glu (19·6 g/kg) to the basal diet. The crude protein equivalent of Gly and Glu was calculated as 119·8 and 59·5 g/100 g amino acid, respectively from National Research Council requirements of poultry(21). The composition of the experimental diets in experiment 2 is shown in Table 2. Chicks were intraperitoneally injected with LPS (0·5 mg/kg body weight) at 21, 23 and 25 d of age, or with Sephadex-G50 (250 mg/kg body weight; Pharmacia, Piscataway, NJ, USA) at age 22 and 24 d to stimulate monocytes/macrophages. Sephadex G-50 superfine (5 g) was dissolved in 100 ml in sterile saline. A blood sample was taken from the brachial vein with a heparinised syringe just before and after 9 and 24 h of the first LPS injection (i.e. 21 d). After blood sampling following the first LPS injection, Sephadex was then injected. Plasma was collected and stored in a manner similar to experiment 1. A blood sample from each bird before the first LPS injection (i.e. 21 d) was used as its own control to calculate changes in parameters for blood acute-phase substances.
Table 2 Composition of the experimental diets in experiment 2

* See Akiba & Matsumoto(Reference Akiba and Matsumoto43).
In experiment 3, the effect of dietary Gly supplementation on the mRNA expression of inflammation-related substances by the liver and spleen following LPS injection was investigated. Forty chicks (age 7 d) were divided into two groups and were provided with the basal diet or diet supplemented with Gly (10 g/kg) for 14 d. The composition of the experimental diets was the same as that in experiment 1. At 21 d of age, chicks were intraperitoneally injected with LPS (1·5 mg/kg body weight). Liver and spleen samples were collected and frozen in liquid N2 from five chicks in each dietary group at 0, 2, 3 and 4 h after LPS injection. Spleen and liver samples were stored at − 80°C until analysed.
All experimental procedures were approved by the ‘The Animal Care and Use Committee’ of the Graduate School of Agriculture of Tohoku University.
Determination of plasma ceruloplasmin and nitrate plus nitrite concentrations
Plasma was separated and stored at − 80°C until assayed. Plasma Cer concentration was determined by the procedure of Sunderman & Nomoto(Reference Sunderman and Nomoto22) using p-phenylenediamine. Acetate buffer (2 ml, 0·1 m; pH 5·4) was added to 0·1 ml plasma sample and pre-incubated for 5 min at 37°C. Thereafter, 1 ml of 27 mm-p-phenylenediamine was added as the substrate for the reaction. The reaction was stopped by adding 50 μl of 1·5 m-sodium azide exactly 30 min later. The resulting colour change was spectrophotometrically measured at 530 nm (Shimazu UVmini1240; Shimazu, Kyoto, Japan). Absorbance of the blank was measured immediately after adding 50 μl of 1·5 m-sodium azide to a mixture of 1 ml of 27 mm-p-phenylenediamine and 0·1 ml plasma. Cer concentration was calculated by the following equation:
where 752 is the molar absorbance coefficient. Plasma NOx concentration, as an indicator of NO production, was estimated by the method of Misko et al. (Reference Misko, Schilling, Salvemini, Moore and Currie23). Briefly, plasma was filtered through a 10 000 Da molecular weight cut-off filter at 4°C and the resultant filtrate incubated with nitrate reductase. The resulting nitrite was reacted with the 2,3-diaminonaphthalene (Dan reagent). Formation of 2,3-diamino-napththotriazole was measured by fluorescence spectrometry (Labsystems Fluoroskan Ascent FL, Osaka, Japan) with excitation at 365 nm and emission read at 450 nm. Plasma parameters, such as Cer and NOx, were expressed as the fold increase following LPS injection compared with their concentrations before the injection.
Quantification of mRNA by real-time-PCR
Total RNA from liver and spleen samples (about 50–100 mg) was extracted by addition of 1 ml of TRIzol-regents (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer's instructions. RNA pellets were washed once with 75 % ethanol, air-dried and dissolved in 20 μl of RNase-free water. RNA concentrations were determined spectrophotometrically at an absorbance of 260 nm, and their integrity checked by electrophoresis (100 V; 20 min) through 0·8 % agarose gels containing ethidium bromide. Total RNA (5 μg) was reverse-transcribed by M-MLV (Invitrogen Corp., Carlsbad, CA, USA) in a 20 μl reaction volume using the oligo-deoxythymidine (dT) 15 primer according to the manufacturer's instructions. cDNA fragments were generated by RT-PCR using the primers as shown in Table 3, and amplification of the genes performed as mentioned below.
Table 3 Primer sequences of selected substances for real-time PCR*

iNOS, inducible NO synthase; TLR, toll-like receptor; TL, TNF-like ligand.
* The specificity of the amplification product was verified by electrophoresis on a 0·8 % agarose-gel following a check of the DNA sequence. cDNA concentration was measured by a spectrophotometer (ND-1000 Spectrophotometer; NanoDrop Technologies, Wilmington, DE, USA).
† National Center for Biotechnology Information accession numbers.
Quantification of selected mRNA species was performed using the cDNA external calibration curve method based on Pfaffl & Hageleit(Reference Pfaffl and Hageleit24) and Overbergh et al. (Reference Overbergh, Valckx, Waer and Mathieu25) in real-time PCR. cDNA standards of selected mRNA species were prepared by the method based on that of Overbergh et al. (Reference Overbergh, Valckx, Waer and Mathieu25) including β-actin as a housekeeping gene. Standard curves were generated using log10-diluted cDNA from pooled total RNA of spleen samples. To normalise the data, the logarithmic-scaled raw data unit cycle threshold was transformed into the linear unit of normalised expression. Real-time PCR was performed using a fluorescence temperature cycler (iCycler Real Time Detection System; Bio-Rad Laboratories, Hercules, CA, USA) and SYBR Green I as a double-stranded DNA-specific binding dye, according to the manufacturer's instructions. Amplifications were carried out using 1·25 U TaKaRa Taq™ or 1·25 U TaKaRa Ex Taq™ (Takara Shuzo, Kyoto, Japan), 0·5 μm of IL-1β, IL-6, TL1A, IFN-γ, iNOS, TLR4 and β-actin primer, 10 × TaKaRa Taq™ buffer (100 mm-2-amino-2-hydroxymethyl-1,3-propanediol-HCl (pH 8·3), 500 mm-KCl, 1·5 mm-MgCl2) or 10 × TaKaRa Ex Taq™ buffer (2·0 mm-MgCl2), 0·05 μl 1:100 diluted SYBR Green I nucleic acid gel stain (BioWhittaker, Inc. Molecular Applications, Rockland, ME, USA), and 1 μl cDNA was used in a total volume of 50 μl. The real-time PCR conditions were: preheat denature at 94°C for 3 min, annealing at 60°C (IL-1β and TL1A), 63°C (IFN-γ), 64°C (iNOS and β-actin) and 65°C (IL-6 and TLR4) for 1 min, and extension at 72°C (IL-6, TL1A, iNOS, IFN-γ and TLR4) and 68°C (IL-1β) for 1 min. SYBR Green I fluorescence was detected at 68 or 72°C at the end of each cycle to monitor the amount of PCR product formed during that cycle. A melting curve analysis of the amplification products was performed at the end of the PCR run, determined by a gradual increase in temperature to 95°C at a rate of 0·05°C/s with continuous measurement of fluorescence to confirm single product generation profiles appropriate to melting temperature. Results are presented as the ratios of IL-1β, IL-6, TL1A, IFN-γ, iNOS and TLR4 to β-actin to correct for differences in the amounts of template DNA used. The abundance of β-actin mRNA as the housekeeping gene was not affected by diet and/or LPS injection in the liver or spleen, as determined in experiment 3 (data not shown).
Statistical analysis
For the analyses, cage replications were considered as the experimental unit. Data were subjected to one-way ANOVA of SAS (1998; SAS Institute, Inc., Cary, NC, USA)(26) and mean values were compared using Duncan's multiple-range test in experiments 1 and 2. The threshold of significance was 0·05. In experiment 1, linear and quadratic contrasts were also employed to identify a possible Gly dose–response effect. In experiment 3, the normal distribution of data was confirmed by the χ2 goodness of fit test. Data were subjected to repeated-measures ANOVA using the MIXED model including Gly treatment, time after LPS injection and the interaction. Data in the same blood sampling time were also compared using Student's t test. The threshold of significance was 0·05.
Results
In experiment 1, the effect of graded supplementation of Gly to the basal diet on plasma acute-phase substances following LPS injection was determined. Results of plasma parameters were expressed as the fold increase induced by LPS injection compared with their value before injection and are summarised in Table 4. Chicks fed the diet supplemented with Gly at 40 g/kg generally showed poor growth performance compared with chicks fed the other diets. Chicks fed the basal diet showed less feed intake for 24 h following LPS injection than chicks fed the diets supplemented with Gly, regardless of the level of Gly supplementation. Increases observed in plasma Cer 24 h after LPS injection in chicks fed the diet supplemented with Gly at 10 or 20 g/kg were lower than those in chicks fed the diet supplemented with Gly at 40 g/kg. Increases in plasma NOx concentrations 9 h after LPS injection in chicks fed the diet supplemented with Gly at 10 g/kg were lower than those in chicks fed the diets supplemented with Gly at 0 or 40 g/kg.
Table 4 Effect of graded supplementation of glycine (Gly; 0, 10, 20 and 40 g/kg) on growth performance, change in feed intake, changes in plasma concentrations of ceruloplasmin (Cer) at 24 h and nitrate plus nitrite (NOx) at 9 h following a single injection of lipopolysaccharide (LPS; 1·5 mg/kg body weight)*
(Mean values with their standard errors for five cages of two birds per dietary group)

a,b,c Mean values (five observations) within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of diets and procedures, see Table 1 and Materials and methods.
† Plasma Cer concentrations before LPS injection in chicks fed the diets supplemented with Gly at 0, 10, 20 and 40 g/kg diet were 19·6 (sem 1·3), 18·6 (sem 1·6), 18·6 (sem 1·8) and 13·7 (sem 1·6) mg/l, respectively.
‡ Plasma NOx concentrations before LPS injection in chicks fed the diets supplemented with Gly at 0, 10, 20 and 40 g/kg diet were 11·5 (sem 0·8), 9·6 (sem 1·1), 12·6 (sem 0·5) and 16·8 (sem 2·2) nmol/ml, respectively.
In experiment 2, the effect of dietary supplementation with Gly on plasma acute-phase substances following LPS injection and growth performance during immunological stimulation was determined under the condition of using isonitrogenous diets. As summarised in Table 5, no significant differences in growth performance among dietary groups were observed before immune stimulation. However, when chicks received immune stimulation, chicks fed the diet supplemented with Gly showed greater body-weight gain and body-weight gain:feed intake ratio than chicks fed the basal diet or the basal diet supplemented with Glu. Plasma concentrations of Cer and NOx were increased by injection of LPS, regardless of dietary treatments. Increases in plasma Cer at 24 h and NOx 9 h after LPS injection in chicks fed the diet supplemented with Gly were lower than those in chicks fed the basal diet or the diet supplemented with Glu.
Table 5 Effect of supplementation with glycine (Gly; 10 g/kg) and glutamic acid (Glu; 19·6 g/kg) on growth performance before and after repeated injections of lipopolysaccharide (LPS) and Sephadex, and changes in plasma concentrations of ceruloplasmin (Cer) at 24 h and nitrate plus nitrite (NOx) at 9 h following the first LPS injection*
(Mean values with their standard errors for six cages of two birds per dietary group)
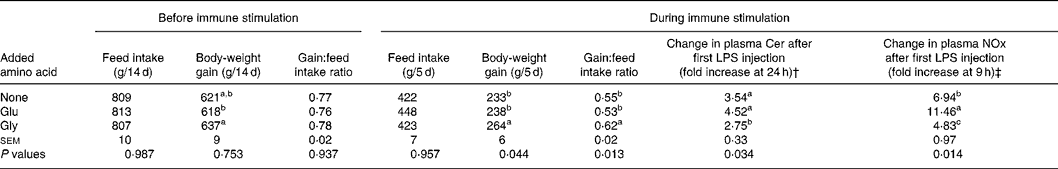
a,b,c Mean values (six observations) within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of diets and procedures, see Table 2 and Materials and methods.
† Plasma Cer concentrations before LPS injection in chicks fed the basal diet and diets supplemented with Gly and Glu were 19 (sem 2), 13 (sem 2) and 11 (sem 1) mg/l, respectively.
‡ Plasma NOx concentration before LPS injection in chicks fed the basal diet and diets supplemented with Gly and Glu were 6·1 (sem 0·6), 6·9 (sem 0·5) and 9·9 (sem 1·9) nmol/ml, respectively.
Fig. 1 summarises the data on mRNA expression of substances related to inflammatory responses in the liver in experiment 3. The highest expression of IL-1β, IL-6 and IFN-γ was observed 2 h after LPS injection and that of iNOS 3 h after LPS injection. Expression of TLR4 mRNA was decreased by LPS injection and this reduction was observed even 4 h after LPS injection. Generally, mRNA expression of these substances in chicks fed the diet supplemented with Gly was lower than those in chicks fed the basal diet after LPS injection. In chicks fed Gly-supplemented diets, a significant reduction was observed in mRNA expression of iNOS at 2 and 4 h, IFN-γ at 4 h and TLR4 at 2 and 4 h following LPS injection.

Fig. 1 Effect of dietary supplementation of glycine (Gly; 10 g/kg diet) on mRNA expression of inflammatory response-related cytokines and substances in the liver of broiler chickens in experiment 3. (a) IL-1, (b) IL-6, (c) interferon (IFN)-γ, (d) inducible NO synthase (iNOS), (e) toll-like receptor (TLR) 4. (○), Basal diet; (●), Gly-supplemented diet. Values are the means of five individual chickens, with standard errors represented by vertical bars. * Mean value was significantly different from that of the chickens fed the basal diet (P < 0·05). Data were also subjected to repeated-measures ANOVA using the MIXED model including Gly treatment, time after LPS injection and the interaction. For IL-1, the effect of Gly treatment was NS (P = 0·460), the effect of time after LPS injection was significant (P = 0·013) and the interaction was NS (P = 0·961). For IL-6, the effect of Gly treatment was NS (P = 0·245), the effect of time after LPS injection was significant (P = 0·001) and the interaction was significant (P = 0·011). For IFN-γ, the effect of Gly treatment was NS (P = 0·138), the effect of time after LPS injection was significant (P = 0·001) and the interaction was NS (P = 0·216). For iNOS, the effect of Gly treatment was NS (P = 0·071), the effect of time after LPS injection was significant (P = 0·001) and the interaction was NS (P = 0·434). For TLR4, the effect of Gly treatment was NS (P = 0·563), the effect of time after LPS injection was significant (P = 0·001) and the interaction was NS (P = 0·062).
Fig. 2 summarises the data on mRNA expression of substances related to inflammatory responses in the spleen in experiment 3. Expression of splenic IL-1β, IL-6, TL1A, iNOS and IFN-γ was markedly enhanced 2 to 3 h after LPS injection and from then on their expression decreased. However, the expression of IL-1β, IL-6, and iNOS 4 h after LPS injection remained higher than those seen before LPS injection. The highest levels of IL-1β, IL-6 and IFN-γ were observed at 2 h and that of iNOS at 3 h after LPS injection. Chicks fed the diet supplemented with Gly had lower mRNA expression of IL-1β at 3 and 4 h, IL-6 at 2 and 3 h, TL1A and iNOS at 2, 3 and 4 h, IFN-γ at 2 and 3 h and of TLR4 at 4 h following LPS injection compared with chicks fed the diet without Gly supplementation.

Fig. 2 Effect of dietary supplementation of glycine (Gly; 10 g/kg diet) on mRNA expression of inflammatory response-related cytokines and substances in the spleen of broiler chickens in experiment 3. (a) IL-1, (b) IL-6, (c) TNF-like ligand (TL) 1A, (d) inducible NO synthase (iNOS), (e) interferon (IFN)-γ, (f) toll-like receptor (TLR) 4. (○), Basal diet; (●), Gly-supplemented diet. Values are the means of five individual chickens, with standard errors represented by vertical bars. * Mean value was significantly different from that of the chickens fed the basal diet (P < 0·05). Data were also subjected to repeated-measures ANOVA using the MIXED model including Gly treatment, time after LPS injection and the interaction. For IL-1, the effect of Gly treatment was significant (P = 0·001), the effect of time after LPS injection was significant (P = 0·001) and the interaction was significant (P = 0·001). For IL-6, the effect of Gly treatment was significant (P = 0·006), the effect of time after LPS injection was significant (P = 0·001) and the interaction was significant (P = 0·009). For TL1A, the effect of Gly treatment was significant (P = 0·001), the effect of time after LPS injection was significant (P = 0·001) and the interaction was significant (P = 0·001). For iNOS, the effect of Gly treatment was significant (P = 0·001), the effect of time after LPS injection was significant (P = 0·001) and the interaction was significant (P = 0·019). For IFN-γ, the effect of Gly treatment was significant (P = 0·011), the effect of time after LPS injection was significant (P = 0·001) and the interaction was NS (P = 0·072). For TLR4, the effect of Gly treatment was NS (P = 0·687), the effect of time after LPS injection was significant (P = 0·005) and the interaction was NS (P = 0·097).
Discussion
TNF-α is an important pro-inflammatory cytokine which is a regulator of host responses to infection, immune responses, inflammation and trauma(Reference Dinarello1–Reference Humphrey and Klasing3). It is well known that acute-phase protein production is induced mainly by pro-inflammatory cytokines such as IL-1, IL-6 and/or TNF-α while NO production is induced by IL-1, TNF-α and/or IFN-γ in mammals(Reference Dinarello1). Thus, pro-inflammatory cytokines are important regulators of metabolic responses in acute-phase inflammation in mammals. In chickens, Cer and NOx are known as acute-phase substances following LPS injection(Reference Chamanza, Veen, Tivapasi and Toussaint27, Reference Takahashi, Orihashi and Akiba28) and plasma concentrations of Cer and NOx were increased by LPS injection(Reference Takimoto, Takahashi, Sato and Akiba18, Reference Chamanza, Veen, Tivapasi and Toussaint27, Reference Takahashi, Orihashi and Akiba28). Increased plasma Cer and NOx as an indicator of NO production (Tables 4 and 5) coincided with the increased levels of IL-1β, IL-6, TL1A, IFN-γ and/or iNOS mRNA expression in the liver and spleen (Figs. 1 and 2) when chicks were challenged with LPS in the present experiment. These findings strengthen the awareness that IL-1β, IL-6, TL1A and/or IFN-γ probably function as pro-inflammatory cytokines in chickens as well as mammals.
The results presented here showed that dietary supplementation with Gly also reduced an increase in the mRNA expression of IL-1β, IL-6, TL1A, IFN-γ, and iNOS in the spleen following LPS injection, and their expression in the liver in some cases (Figs. 1 and 2). Gly supplementation also lowered the increased ratio of plasma Cer and/or NOx that following LPS injection (Tables 4 and 5). It has been previously reported that inflammatory responses following LPS injection are modulated by dietary protein concentration in chickens(Reference Takahashi, Yodogawa and Akiba29). However, the effect of supplemental Gly as observed in the present experiment was independent of an increase in the protein concentration in the diet, since chicks fed the diet with Gly showed lower increases in plasma Cer and NOx than chicks fed the diet with Glu (Table 4). A reduced inflammatory response in birds fed a Gly-supplemented diet can be explained by the modulation of TNF-α and IL-1β and IL-10 secretion in mammals(Reference Grimble, Jackson, Persaud, Wride, Deleres and Engler4–Reference You, Wang, Li, Chen, Liu and Gong13). The present results suggest that the changes in the mRNA expression of IL-1β, IL-6, TL1A and/or IFN-γ by dietary supplementation with Gly are related to modulation of the inflammatory response following LPS injection in chickens. The inhibitory effect of dietary Gly on pro-inflammatory cytokine production and expression reported in mammals was demonstrated using animals fed a diet containing Gly at 50 g/kg diet(Reference Ikejima, Iimuro, Forman and Thurman5, Reference Wheeler, Rose, Yamashima, Enomoto, Seabra, Madren and Thurman7, Reference Mauriz, Matilla, Culebras, Gonzalez and Gonzalez-Gallego9, Reference Neyrinck, Margagliotti and Delzenne11, Reference You, Wang, Li, Chen, Liu and Gong13). However, it seems that the effect of Gly in chicks occurs with Gly at less than 40 g/kg diet.
TLR are major components of the pattern-recognition receptor that detect invading micro-organisms by molecular structures known as its associated molecular patterns(Reference Takeda, Kaisho and Akira30). The importance of TLR function is evident by their role in the mammalian immune system(Reference Takeda, Kaisho and Akira30). Upon activation with an appropriate ligand, TLR induce a range of responses including the production of cytokines. Signalling through TLR leads to widespread induction of the cellular components of the innate and adaptive immune system. TLR4 demonstrates a distinct specificity for the LPS of gram-negative bacteria(Reference Takeda, Kaisho and Akira30). It has been reported that administration of LPS decreased the levels of TLR4 mRNA in mammalian liver and spleen(Reference Lang, Silvis, Desphande, Nystrom and Frost31). You et al. (Reference You, Wang, Li, Chen, Liu and Gong13) observed that attenuation of LPS-induced liver injury in mice by pretreatment with Gly may be associated with its role in down-regulating TLR4 expression. In chicks, it has been reported that TLR4 is present in a broad range of tissue including the liver and spleen(Reference Iqbal, Philbin and Smith32). LPS injection resulted in a 50–70 % reduction of mRNA expression of TLR4 in the liver and spleen relative to non-injected controls. Reduction in TLR4 mRNA expression was also observed in chicks fed the diet supplemented with Gly in the present experiment. Collectively, reduced TLR4 mRNA expression following LPS injection by dietary supplementation with Gly may be associated with the modulation of inflammatory responses, as observed in the present experiment.
Recently, Dahiya et al. (Reference Dahiya, Hoehler, Wilkie, Van Kessel and Drew33, Reference Dahiya, Hoehler, Van Kessel and Drew34) observed that high levels (over 30·0 g/kg diet) of dietary Gly resulted in high Clostridium perfringens colonisation and high intestinal lesion scores when chicks were orally challenged with C. perfringens. They concluded that Gly in the diet of chicks is an important determinant of which are nearly ubiquitous gram-positive, spore-forming, prolific, toxigenic anaerobic bacteria. Gly at less than 30 g/kg diet partly alleviated enhancement of the systemic inflammatory response induced by LPS injection in the present study. Therefore, it would appear that the effect of dietary Gly on systemic inflammation is different from that on intestinal inflammation. In addition, crystalline Gly was used as the supplemental Gly source in the present study, while encapsulated Gly was used in the experiment of Dahiya et al. (Reference Dahiya, Hoehler, Van Kessel and Drew34). Hence, the Gly source used may be another factor modulating the effect of Gly on inflammation. Furthermore, it has been considered that the availability of dietary Gly may be limited in early nutrition, if the crude protein concentration of a diet is low, and vegetable ingredients are primarily used(Reference Corzo, Kidd, Burnham and Kerr35, Reference Dean, Bidner and Southern36). Further experiments are required to optimise dietary Gly (or Gly+Ser) level for maximising animal health and production.
Exposure of animals to infectious or inflammatory agents induces not only immune responses in the host, but also causes metabolic changes that lead to decreased rates in body-weight gain and feed consumption(Reference Koutsos, Klasing, Garnsworthy and Wiseman2, Reference Humphrey and Klasing3, Reference Roura, Homedes and Klasing37). The combination of sheep erythrocytes and Sephadex gave mostly an additive change in chicken growth rates(Reference Klasing, Laurin, Peng and Fry38, Reference Takahashi, Akiba and Tamura39). We also found a similar effect of the combination of LPS and Sephadex on changes in chicken growth performance(Reference Misko, Schilling, Salvemini, Moore and Currie23). Therefore, we used this model system to determine changes in growth performance during immunological stimulation. It is established that anorexia is a primary outcome of the acute-phase inflammatory response affecting performance(Reference Klasing, Laurin, Peng and Fry38). In the present study, the effect of dietary Gly supplementation on growth performance was limited to improved feed utilisation during the inflammatory response (Table 4, experiment 2) and to improved feed intake for 24 h following a single injection of LPS (Table 5, experiment 1). It would appear that dietary Gly supplementation above levels recommended for growth may be a way to alleviate the reduction in growth performance observed during the inflammatory response. However, the results of the growth-performance data obtained in the present experiment are based on observations of only a small number of animals. Further experiments are required to prove if dietary Gly supplementation improves growth performance during immunological stimulation.
Glu was supplemented at relatively high doses to achieve an isonitrogenous diets in experiment 2. This may have resulted in detrimental over-supplemented effects of Glu, since excessive intake of a single amino acid above the requirement has unfavourable effects on chicks performance(Reference Edmonds and Baker40, Reference Baker41). However, growth performance in chicks fed the Glu diet was comparable with that seen in chicks fed the control diet both before and during immune stimulation (Table 4). Our previous experiment indicated that addition of Glu at 2 g/100 g diet did not affect growth performance in broiler chicks(Reference Takahashi, Konash, Akiba and Horiguchi42). Furthermore, Baker(Reference Baker41) noted that an excessive intake of Glu seems to be well tolerated in pigs. Thus, dietary supplementation of Glu at the level used in the present experiment did not have detrimental effect at least upon the growth performance of broiler chicks.
In conclusion, the present results suggest that dietary Gly supplementation above levels recommended for growth reduced the inflammatory response and associated growth retardation seen in response to systemic challenge. Changes in TLR4 expression induced by supplemental dietary Gly may indicate one of its possible mechanisms of action.
Acknowledgements
The present study was in part supported by a Grant-in-Aid for Scientific Research (C) to K. T. (no. 17580232) from the Ministry of Education, Science, Culture, Sports and Technology of Japan, and by the Secure and Healthy Livestock Farming Project from the Ministry of Agriculture, Forestry and Fisheries of Japan. The authors report no conflict of interest in the preparation of this paper. We thank Dr Ahmad Mujahid (Kyushu University, Fuoka, Japan) for English revision in this paper.











