Clostridium perfringens (CP) is a gram-positive, anaerobic and opportunistic pathogen. CP overgrowth and the exotoxins it produces are responsible for the outbreak of necrotic enteritis (NE) in some cases. NE is an inflammatory disease of the small intestine affecting poultry, resulting in the loss of over 2 billion dollars annually in the international broiler industry( Reference Van der Sluis 1 ). In recent years, NE incidence has been increasing because of the limited use or removal of antibiotics. As reviewed by Shojadoost et al. ( Reference Shojadoost, Vince and Prescott 2 ), several risk factors predispose an individual to the onset of NE, including coccidial infection, barley- or wheat-based diet, high animal protein diet and a novel pore-forming toxin, NE B-like toxin. The most important known predisposing factor for NE is intestinal damage caused by coccidial pathogens( Reference Shojadoost, Vince and Prescott 2 , Reference Williams 3 ). NE manifestation includes acute clinical disease and subclinical disease( Reference Shojadoost, Vince and Prescott 2 , Reference Caly, D’Inca and Auclair 4 ). Unlike clinical infection course with typical symptoms and high mortality, subclinical infection is difficult to detect and results in greater economic losses by chronic intestinal mucosal damage( Reference Caly, D’Inca and Auclair 4 ).
Previous studies have demonstrated that chickens challenged with both CP and coccidial vaccine have higher numbers of CP in the intestine than healthy chickens or those administered either CP or coccidial vaccine alone( Reference Pedersen, Bjerrum and Heuer 5 , Reference Park, Lillehoj and Allen 6 ). CP overgrowth is known to stimulate gene transcripts encoding T helper 1 (Th1) and Th2 cytokines such as IL-1β, IL-10, IL-4, interferon-α (IFN- α) and IFN- γ ( Reference Park, Lillehoj and Allen 6 , Reference Collier, Hofacre and Payne 7 ). Furthermore, cytokines mediate tight junction (TJ) barrier function disorders, resulting in immune perturbation and inflammatory response, ultimately promoting the initiation and/or development of intestinal and systemic diseases( Reference Suzuki 8 ). CP infection has been reported to decrease the mRNA expression of intestinal TJ( Reference Liu, Guo and Guo 9 , Reference Du, Wang and Gan 10 ) and the infection is associated with proteolytic capacity towards TJ proteins( Reference Pruteanu and Shanahan 11 ). However, factors such as nutrients also influence intestinal integrity, and the use of arginine has received increasing attention because of its role in immune regulation and its ability to restore mucosal integrity.
l-Arginine is a functional amino acid that plays a significant role in several physiological processes via its metabolites such as nitric oxide (NO) or polyamine. Dietary arginine supplementation reinforces the immune status of animals, consequently lowering morbidity and mortality in infectious disease( Reference Field, Johnson and Pratt 12 , Reference Li, Yin and Li 13 ). l-arginine is known to promote the proliferation of lymphocytes in Peyer’s patches, balance pro-inflammatory (IFN- γ and IL-2) and anti-inflammatory (IL-4 and IL-10) cytokines and increase the secretory IgA (sIgA) level in burn-injured mice( Reference Fan, Meng and Guo 14 ). Moreover, studies in several animal models have revealed that l-arginine supplementation is capable of reducing intestinal mucosal lesions, maintaining intestinal barrier function, ameliorating mucosal morphology and promoting enterocyte proliferation( Reference Viana, Santos and Generoso 15 – Reference Koppelmann, Pollak and Mogilner 18 ).
The benefits of l-arginine supplementation on animals seem to be dose dependent. Peck et al. ( Reference Peck, Babcock and Alexander 19 ) fed mice with severe protein malnutrition normal protein diets with different l-arginine concentrations and studied the mortality of these mice following Salmonella typhimurium. Their results revealed that mortality of mice after infection did not reduce by supplementation with 10–20 g/kg l-arginine, but mice mortality was aggravated by supplementation with 50 g/kg l-arginine. Zhan et al. ( Reference Zhan, Ou and Piao 20 ) reported that a lower level of dietary arginine (1·52 %) improved microvascular development in the small intestines of early-weaned pigs, but at a higher concentration of dietary arginine (2·02 %) adverse effects such as gut dysfunction were observed. However, it remains to be clarified whether or not dietary l-arginine concentrations higher than the National Research Council (NRC, 1994)( 21 )-recommended level would benefit NE broilers. Therefore, we carried out the present study to investigate the effects of dietary l-arginine level higher than those recommended by the NRC (1994)( 21 ) on the mucosal integrity of intestines, microbial flora and mucosal immunity of broilers in a subclinical NE model.
Methods
In vivo study
Experimental design and diets
All experimental procedures were approved by the Animal Care and Use Committee of China Agricultural University. A total of 210 1-d-old Arbor Acres female broiler chicks were randomly allotted to six treatment groups (n 35/group). A 3×2 factorial randomised complete block design was used to determine the effects of dietary treatments (dietary l-arginine level and feeding duration), pathogen exposure (co-infected with or without Eimeria and CP) and their interactions. The birds were divided into the following three dietary treatment groups: CON, birds in this group were fed a basal diet from days 1 to 28; CON/ARG, birds were given a basal diet for the first 8 d and a high-arginine diet from days 9 (1 d before challenge) to 28; ARG, birds received a high-arginine diet from days 1 to 28. In the high-arginine diet, the amount of l-arginine added to the basal diet accounted for 1·87 % of the diet, and the alanine concentration in the diet was reduced for compensation. Wheat-based meal diets were formulated to meet or exceed the NRC (1994)( 21 ) requirements. The birds were fed ad libitum and each treatment group was housed in a separate rearing isolator. Each isolator had a floor space of 11 200 (160×70) cm2, and was equipped with two nipple drinkers and one feeder. Table 1 presents the ingredients and nutrition level of the diet. The arginine used was of reagent grade with a purity of over 98 % (A5006; Sigma-Aldrich Co.). Dietary amino acids were analysed by HPLC. All diets were pelleted and crumbled.
Table 1 Composition and nutrient levels of experimental diets

* Supplied the following per kg complete feed: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0·15 mg; I, 0·35 mg.
† Supplied the following per kg complete feed: retinyl acetate, 24 mg; cholecalciferol, 6 mg; menadione, 2·65 mg; thiamine, 2 mg; riboflavin, 6 mg; cyanocobalamin, 0·025 mg; α-tocopheryl acetate, 20 mg; biotin, 0·0325 mg; folic acid, 1·25 mg; pantothenic acid, 12 mg; niacin, 50 mg.
‡ Calculated value.
§ Analysed concentrations.
Eimeria and Clostridium perfringens co-infection procedures
The Eimeria and C. perfringens (EM/CP) challenge was performed on the basis of the reports of Collier et al. ( Reference Collier, Hofacre and Payne 7 ) and Liu et al. ( Reference Liu, Guo and Wang 22 ), with some modifications. Briefly, 10-d-old chickens in the infected groups were each challenged with live coccidiosis vaccine (2×104 oocysts containing a mixture of Eimeria acervulina, Eimeria maxima and Eimeria tenella) suspended in 500 μl of normal saline by oral gavage. From days 14 to 20, the infected groups were orally challenged with 1 ml of C. perfringens type A culture broth at 1×108 colony-forming units (CFU)/bird per d. Chickens in the uninfected group received the same amount of normal saline and sterile broth culture medium at the corresponding times. Coccidiosis vaccine was provided by Zhengdian Biotechnology Company, and the CP strain (CVCC2027) was obtained from the China Veterinary Culture Collection Center.
Sample collection
On days 21 and 28, eight birds were randomly selected per treatment and killed by jugular exsanguination. The midregions of the jejunum and ileum (approximately 1 cm) were collected, rapidly frozen in liquid N2 and stored at –80°C for mRNA analysis. The digesta from the ileum (from the midpoint of the ileum to 1 cm proximal to the ileocaecal junction) and caecum were aseptically collected and stored at –20°C for bacteria counting. In addition, mucosa for the total sIgA examination was scraped from the bottom half of the jejunum and ileum, and stored at –20°C.
Plasma d-xylose concentrations
On days 21 and 28, another eight feed-deprived broilers from each group were individually weighed and administered d-xylose (X1500; Sigma-Aldrich Co.) solution at a dose of 0·1 g/kg body weight (infused with 10 % d-xylose at 1 ml/kg body weight) by oral gavage, as described by Hou et al. ( Reference Hou, Wang and Zhang 23 ). A period of 1 h later, blood samples were collected into heparinised vacuum tubes by wing vein puncture. Plasma was separated by centrifugation at 3000 rpm for 10 min at 4°C and then stored at –20°C. The d-xylose concentration was determined using the d-xylose assay kit (A035; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
Ussing chamber experiment
The Ussing chamber procedures were performed as described by Clarke( Reference Clarke 24 ) with some modifications. On days 21 and 28 of the trial, after blood samples were obtained from the d-xylose-treated birds, they were then anaesthetised with 5 % (w/w) pentobarbital Na solution injection into the wing vein. The stomachs were then ripped open, and 5-cm-long ileal fragments proximal to the ileocaecal junction were rapidly separated, opened longitudinally and washed in ice-cold oxygenated (95 % O2/5 %CO2) Krebs–Ringer buffer( Reference Clarke 24 ). Serosal layers were stripped off, and the ileal mucosa was mounted on the Ussing chamber system (model VCC MC6; Physiologic Instruments) with an exposed area of 0·2 cm2. Mucosal and serosal sides were equilibrated in 5 ml of oxygenated (95 % O2/5 % CO2) Krebs–Ringer buffer maintained at 37°C for 20 min, and then the medium was removed from both chambers. Fluorescein isothiocyanate dextran 4 kDa (FD4, 4000 MW; Sigma-Aldrich Co.) was diluted with 5 ml of preheated (37°C) Krebs–Ringer buffer to 2 mg/ml and added to the mucosal side. The serosal side was replaced with the diluent. FD4 transportation was monitored by sampling 100 µl of solution from the serosal compartment after 30 min. FD4 concentration was measured in a fluorescence microplate reader (FLx800; Bio-Tek Instruments, Inc.), and mucosal-to-serosal flux of FD4 was expressed as µg/cm2 per·h.
Measurement of secretory IgA in intestinal mucosa
Mucosal samples (0·3 g) were placed in tubes containing 2·7 ml of normal saline, and the mixtures were homogenised. The supernatants were collected by centrifugation at 4000 rpm for 10 min at 4°C. The total sIgA concentration was measured using a Chicken IgA ELISA Quantification Set (E30-103; Bethyl Laboratories Inc.) according to the manufacturer’s instructions. The protein concentration in the supernatant was measured using a BCA protein quantification kit (CW0014S; CW Bio). Values were expressed as the Ig level per gram of protein.
Ileal and caecal microflora analysis
The intestinal populations of lactobacilli, Escherichia coli and CP were analysed using 16S rDNA primers by quantitative PCR, as described previously( Reference Du, Gan and Li 25 ). In brief, genomic DNA from the intestinal contents was used as a template for PCR amplification using SYBR® Premix Ex TaqTM (RR420A; TaKaRa) and an ABI 7500 fluorescence quantitative PCR instrument (Applied Biosystems). Absolute quantification was achieved using standard curves constructed by amplification of known amounts of target plasmid DNA. The mathematical formula to calculate the number of target gene copies was referred to the method described by Du et al.( Reference Du, Gan and Li 25 ). Bacterial DNA was extracted using a QIAamp DNA Stool Mini Kit (51604; Qiagen), according to the manufacturer’s instructions. The targeted groups, primer sequences, amplicon sizes and literature referenced are shown in Table 2. The CT of unknown samples was determined and compared with the standard curves. The results were expressed as log10 gene copies per gram (wet weight) of intestinal contents.
Table 2 16S rDNA real-time PCR primers used for microflora analysis
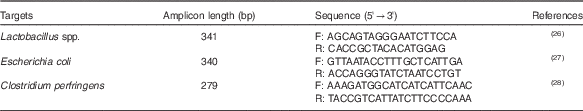
F, forward; R, reverse.
Real-time quantitative PCR
Total RNA was extracted from intestinal tissues using Trizol reagent (15596018; Invitrogen Life Technologies) according to the manufacturer’s protocol. The concentration and purity of RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific). In brief, 1 µg of total RNA from each sample was reverse-transcribed into complementary DNA (cDNA) using a PrimeScript RT reagent kit with cDNA eraser (RR036A; TaKaRa). One-step real-time PCR was performed with the SYBR® Premix Ex TaqTM (RR420A) using an ABI 7500 fluorescence quantitative PCR instrument in accordance with the manufacturer’s guidelines. Table 3 lists the quantitative real-time PCR primers used in our study. The relative mRNA expression levels of each target gene were calculated based on the expression of the housekeeping gene GAPDH using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
29
).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
29
).
Table 3 Primers used for quantitative real-time PCR

ZO-1, zonula occludens 1; IFN-γ, interferon-γ; TLR2, toll-like receptor 2; NOD1, nucleotide-binding oligomerisation domain 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In vitro study
Determination of antimicrobial activity of nitric oxide against Clostridium perfringens
We measured the direct antimicrobial action of NO towards CP according to the previous study( Reference Du, Gan and Li 25 ), with some modifications. In brief, 1×105 CFU of CP was mixed with 1 mL of fluid thioglycollate (FT) medium (CM801; Beijing Luqiao technology Co., Ltd) in sterile centrifuge tubes containing 0, 125, 250, 500, 1000, 2000 or 4000 μmol/l S-nitroso-N-acety1-N-dl-penicillamine (SNAP, N3398; Sigma-Aldrich Co.) as the NO source. The negative control group consisted of 1 ml of FT medium without CP and SNAP. After anaerobic incubation at 37°C for 24 h, 200 μl of culture medium was added to the microwell plate. The bacteria were counted by measuring the optical density of the culture at 600 nm using a microwell plate reader (ELx800; Bio-Tek Instruments, Inc.).
Statistical analysis
In vivo data were analysed using the General Linear Model procedure in SPSS version 18.0 (SPSS Inc.), and subjected to two-way ANOVA in a 3×2 factorial arrangement to analyse the main effects of dietary treatments and challenge, and their interaction. One-way ANOVA and Duncan’s multiple comparison were used when a significant interaction was observed. When the main effect of dietary treatments was significant, polynomial contrasts were performed over the main effect means for dietary treatments (averaged over CP challenge). In vitro data were analysed with one-way ANOVA and Duncan’s multiple comparison analysis. Results are presented as the means with pooled standard errors. All statements of significance were based on P<0·05.
Results
Plasma d-xylose concentration and ileal fluorescein isothiocyanate dextran 4kDa flux
As shown in Fig. 1(A) and (B), the plasma d-xylose concentration was significantly lower in the pathogen-challenged groups than in the uninfected groups on days 21 and 28 (P<0·01). Furthermore, the plasma d-xylose concentration was significantly higher in the ARG group than in the CON group on day 21, regardless of pathogen challenge (P<0·05). There was no interaction between EM/CP co-infection and dietary treatments on the plasma d-xylose concentration on days 21 and 28 (P>0·05). EM/CP co-infection enhanced the ileal FD4 flux on days 21 and 28 (P=0·032 and 0·071, respectively; Fig. 1(C) and (D)). Dietary l-arginine supplementation significantly reduced the ileal FD4 flux on day 21 (P<0·05).

Fig. 1 Effect of dietary l-arginine level and feeding duration on plasma d-xylose concentration (mmol/l, A and B) and ileal fluorescein isothiocyanate dextran 4kDa (FD4) flux (µg/cm2·per h, C and D). Values are means (n 8), with their standard errors represented by vertical bars. Unchallenged, chickens without EM/CP co-infection; challenged, chickens with EM/CP co-infection; ![]() , CON, basal diet from days 1 to 28;
, CON, basal diet from days 1 to 28; ![]() , CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28;
, CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28; ![]() , ARG, l-arginine diet from days 1 to 28. a,b Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
, ARG, l-arginine diet from days 1 to 28. a,b Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
Intestinal secretory IgA level
The ileal sIgA level on day 21 was obviously higher in infected birds than in uninfected birds (P<0·05); however, they decreased in the infected groups on day 28 (P<0·05; Fig. 2). In addition, the jejunal sIgA level on day 28 was significantly higher in the ARG group than in the CON and CON/ARG groups (P=0·01). No interactions on the sIgA levels were found between dietary treatments and pathogens challenge on days 21 and 28 (P>0·05).
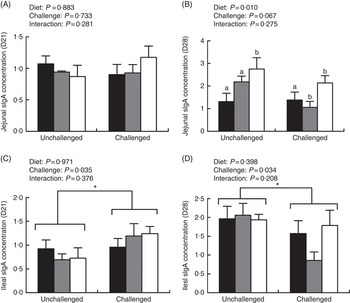
Fig. 2 Effect of dietary l-arginine level and feeding duration on secretory IgA (sIgA) concentration (mg/g protein) of jejunal (A and B) and ileal (C and D) mucosa. Values are means (n 8), with their standard errors represented by vertical bars. Unchallenged, chickens without EM/CP co-infection; challenged, chickens with EM/CP co-infection; ![]() , CON, basal diet from days 1 to 28;
, CON, basal diet from days 1 to 28; ![]() , CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28;
, CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28; ![]() , ARG, l-arginine diet from days 1 to 28. a,b Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
, ARG, l-arginine diet from days 1 to 28. a,b Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
Quantification of ileal and caecal microbiota
Birds co-infected with EM/CP had higher populations of E. coli and CP in the caecal contents on day 21 (P<0·05 and P<0·001, respectively; Fig. 3). Regardless of pathogen challenge, addition of l-arginine (ARG and CON/ARG groups) significantly decreased the population of CP in the ileum (P<0·001). On day 21, dietary treatments and EM/CP co-infection exerted an interactive effect on the population of CP in the ileal contents (P<0·05). In unchallenged birds, the CP population was decreased in the CON/ARG and ARG groups compared with the CON group, whereas in challenged birds it was the lowest in the ARG group with no significant differences between the other two groups. Moreover, there was an interaction between dietary treatments and pathogen challenge on the caecal CP population (P<0·05): l-arginine supplementation significantly decreased the CP population in the caecum of EM/CP-challenged birds. However, different dietary treatments, pathogen challenge or interaction of these two factors did not result in changes in the Lactobacillus populations (P>0·05).

Fig. 3 Effect of dietary l-arginine level and feeding duration on microbial populations (log10 gene copies/g wet weight) in ileal (A, B and C) and caecal (D, E and F) contents on day 21. Values are means (n 8), with their standard errors represented by vertical bars. Unchallenged, chickens without EM/CP co-infection; challenged, chickens with EM/CP co-infection; ![]() , CON, basal diet from days 1 to 28;
, CON, basal diet from days 1 to 28; ![]() , CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28;
, CON/ARG, basal diet from days 1 to 8 and l-arginine diet from days 9 to 28; ![]() , ARG, l-arginine diet from days 1 to 28. a,b,c Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
, ARG, l-arginine diet from days 1 to 28. a,b,c Mean values with unlike letters were significantly different (P<0·05). * Significant main effect (P<0·05) of Emeria and Clostridium perfringens (EM/CP) co-infection.
Gene expression of tight junction proteins
On day 21, EM/CP challenge significantly down-regulated the mRNA expression levels of jejunal and ileal claudin-1 and jejunal occludin (P<0·05), but increased the ileal claudin-2 mRNA expression (P<0·05; Tables 4 and 5). In addition, l-arginine expression significantly increased the jejunal claudin-1 expression on day 21 and the ileal occludin expression on day 28, regardless of challenge (P<0·05). Furthermore, the interactions of dietary treatments and pathogen challenge were found to significantly affect jejunal claudin-2 and ileal occludin expression on day 28 (P<0·05). In the co-infected birds, the mRNA expression of claudin-2 and occludin was elevated with l-arginine supplementation (P<0·05). However, no significant differences were seen on the expression of zonula occludens 1 (ZO-1) by the main effects and their interaction on days 21 and 28.
Table 4 Effect of dietary l-arginine level and feeding duration on the relative mRNA expression of the jejunal tight junction (Mean values with their standard errors; n 8)

ZO-1, zonula occludens 1; unchallenged, chickens without Eimeria and Clostridium perfringens (EM/CP) co-infection; challenged, chickens with EM/CP co-infection; CON, basal diet from days 1 to 28; CON/ARG, basal diet from days 1 to 8 and high-arginine diet from days 9 to 28; ARG, high-arginine diet from days 1 to 28; Arg×Challenge, interaction between dietary l-arginine treatment and EM/CP co-infection.
a,b Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
Table 5 Effect of dietary l-arginine level and feeding duration on the relative mRNA expression of the ileal tight junction (Mean values with their standard errors; n 8)

ZO-1, zonula occludens 1; unchallenged, chickens without Eimeria and Clostridium perfringens (EM/CP) co-infection; challenged, chickens with EM/CP co-infection; CON, basal diet from days 1 to 28; CON/ARG, basal diet from days 1 to 8 and high-arginine diet from days 9 to 28; ARG, high-arginine diet from days 1 to 28; Arg×Challenge, interaction between dietary l-arginine treatment and EM/CP co-infection.
a,b,c Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
Inflammation-related gene expression
As shown in Tables 6 and 7, co-infection with EM/CP significantly elevated the mRNA expression levels of jejunal IFN- γ on days 21 and 28 (P<0·05), ileal IFN- γ on day 28 (P<0·01), jejunal toll-like receptor 2 (TLR2) on days 21 and 28 (P<0·05) and jejunal nucleotide-binding oligomerisation domain 1 (NOD1) on day 28 (P<0·05). Regardless of challenge, l-arginine supplementation increased the mRNA expression levels of jejunal and ileal IFN-γ on day 28 (P<0·001 and P<0·01, respectively), jejunal IL-10 on day 28 (P<0·05) and ileal IL-10 on day 21 (P<0·05).
Table 6 Effect of dietary l-arginine level and feeding duration on the relative mRNA expression of the jejunal tight junction and immune-related molecules (Mean values with their standard errors; n 8)

IFN- γ, interferon-γ; TLR2, toll-like receptor 2; NOD1, nucleotide-binding oligomerisation domain 1; unchallenged, chickens without Eimeria and Clostridium perfringens (EM/CP) co-infection; challenged, chickens with EM/CP co-infection; CON, basal diet from days 1 to 28; CON/ARG, basal diet from days 1 to 8 and high-arginine diet from days 9 to 28; ARG, high-arginine diet from days 1 to 28; Arg×Challenge, interaction between dietary l-arginine treatment and EM/CP co-infection.
a,b,c Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
Table 7 Effect of dietary l-arginine level and feeding duration on the relative mRNA expression of the ileal tight junction and immune-related molecules (Mean values with their standard errors; n 8)

IFN- γ, interferon-γ; TLR2, toll-like receptor 2; NOD1, nucleotide-binding oligomerisation domain 1; unchallenged, chickens without Eimeria and Clostridium perfringens (EM/CP) co-infection; challenged, chickens with EM/CP co-infection; CON, basal diet from days 1 to 28; CON/ARG, basal diet from days 1 to 8 and high-arginine diet from days 9 to 28; ARG, high-arginine diet from days 1 to 28; Arg×Challenge, interaction between dietary l-arginine treatment and EM/CP co-infection.
a,b Mean values in the same row with unlike superscript letters were significantly different (P<0·05).
There was a significant interaction between dietary treatments and EM/CP challenge on the mRNA expression of IFN- γ in the ileum on day 28 (P<0·05). The IFN- γ mRNA expression in challenged birds was significantly increased with l-arginine supplementation (P<0·05). Furthermore, the interaction between dietary treatments and EM/CP challenge significantly influenced NOD1 mRNA expression in the jejunum on day 21 and in the ileum on day 28 (P<0·05). l-Arginine supplementation significantly increased the NOD1 mRNA expression in chickens challenged by EM/CP (P<0·05). In addition, jejunal IL-10 mRNA expression was significantly influenced by the interaction between dietary treatments and pathogen challenge on day 28 (P<0·05). The IL-10 mRNA expression was the lowest in the CON group co-infected with EM/CP and the highest in the ARG groups (P<0·05).
In vitro cytotoxicity of nitric oxide towards Clostridium perfringens
There were no significant differences in the optical density at 600 nm among the different treatment groups at the beginning of the experiment (data not shown). After incubation for 24 h at 37°C, CP growth was completely inhibited by 2000 and 4000 μm of SNAP (P<0·001, Fig. 4).

Fig. 4 Direct antimicrobial activity of nitric oxide towards Clostridium perfringens (CP). Bacteria were cultured in fluid thioglycollate medium in the presence of 0, 125–4000 μmol/l S-nitroso-N-acetylpenicillamine (SNAP), as described in the ‘Methods’ section. After 24 h, bacterial growth was monitored by measuring the optical density at 600 nm. Values are means (n 3), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05).
Discussion
Cereals with high level of NSP (wheat, rye and barley), and coccidial infection, favour the proliferation of CP and its toxin production( Reference Shojadoost, Vince and Prescott 2 ). Here, we induced experimental NE in broiler chickens using wheat-based diets and challenge with Emeria and CP. Although no obvious clinical signs of NE or NE-related mortality were observed, the challenge did induce the macroscopic pathological changes in the intestine, such as distinct hyperaemia, haemorrhage and focal necrosis or ulceration. In addition, pathogen challenge resulted in impaired intestinal absorption, increased ileal mucosal permeability and high colonisation rates of CP in the caecum, which were in accordance with the characteristics of subclinical NE established in previous studies( Reference Liu, Guo and Wang 22 , Reference Gholamiandehkordi, Timbermont and Lanckriet 30 ).
The intact intestinal tract is not only essential for nutrition absorption but also functions as a physical barrier against the harmful components. d-Xylose absorption, mucosal permeability and expression of TJ proteins are the widely used indicators of intestinal epithelial health. As d-xylose crosses the intestinal mucosa via an Na+-dependent mobile-carrier mechanism, active absorption of d-xylose indicates active absorption of water and Na( Reference Doerfler, Cain and Edens 31 ). In the case of malabsorption syndrome, the entry of d-xylose from the gut lumen to the portal vein is damaged, resulting in reduced concentrations of d-xylose in the blood( Reference Doerfler, Cain and Edens 31 , Reference Mansoori, Nodeh and Modirsanei 32 ). In the present study, decreased plasma d-xylose concentration in pathogen-challenged birds reflected malabsorption of the small intestine, which could be related to the impaired intestinal epithelium. The addition of l-arginine increased the plasma d-xylose concentrations regardless of EM/CP challenge, indicating that l-arginine could promote the ability of the small intestine to absorb water and Na. This was consistent with previous results in rats, which reported that l-arginine (1 mm) added to the oral rehydration solutions could improve intestinal water and Na absorption( Reference Wapnir, Wingertzahn and Teichberg 33 ). In this study, we used FD4 flux determined by the Ussing chamber to evaluate intestinal permeability. FD4 permeates the intestinal epithelium mainly via paracellular pathways( Reference Hamard, Mazurais and Boudry 34 ). When the intestinal barrier is impaired, FD4 flux from the mucosal to serosal side will increase. In this study, the remarkable elevation of FD4 flux in the ileum of challenged birds indicated impaired intestinal barrier function. l-Arginine administration reduced the ileal FD4 flux regardless of pathogen challenge, revealing that dietary l-arginine exerts a protective effect on intestinal barrier function.
TJ proteins are vital for the regulation of gut barrier function. During inflammation, the disrupted TJ may result in increased mucosal permeability and pathogen invasion( Reference Turner 35 ). Following EM/CP challenge, changes were discovered in intestinal TJ expression, characterised by the reduced claudin-1 and occludin mRNA expression levels, and even elevated claudin-2 expressions. Claudin-1 and occludin are barrier-forming TJ proteins, whereas claudin-2 is a pore-forming TJ protein( Reference Suzuki 8 ). Thus far, the up-regulation of claudin-2 expression has been only consistently reported in inflammatory bowel disease, and it is regarded as an indicator of inflammation( Reference Turner 35 ). Occludin exerts many regulatory functions and is sensitive to changes in the TJ( Reference Feldman, Mullin and Ryan 36 ). The barrier created by occludin and the adjacent cells prevents macromolecules from passing through it, but allows small ions to pass through( Reference Al-Sadi, Khatib and Guo 37 ). Our study revealed that l-arginine prevented the EM/CP-challenge-induced decrease in claudin-1 mRNA expression and increased the occludin mRNA expression, which could be conducive to the restoration of intestinal permeability. This finding was similar to those reported by previous studies( Reference Beutheu, Ouelaa and Guérin 38 , Reference Ren, Yin and Wu 39 ). In the present study, an elevation of claudin-2 expression in challenged birds fed a high-arginine diet unexpectedly did not lead to increased paracellular permeability, which could probably be attributed to increased expression of tightening TJ proteins in the meantime.
As gut microbial composition is one of the indicators of gut health( Reference M’Sadeq, Wu and Swick 40 ), bacteria can contribute to enhanced intestinal permeability if the gastrointestinal ecosystem is imbalanced( Reference Lu, Wu and Xu 41 ). Therefore, we estimated the populations of Lactobacillus, E. coli and CP in the digesta. Many specific members of microflora have been found to coexist with NE, of which the proportion of E. coli is reported to be one of the largest( Reference Collier, Hofacre and Payne 7 , Reference Jia, Slominski and Bruce 42 , Reference Mcreynolds, Byrd and Anderson 43 ). Similar to previous studies( Reference Liu, Guo and Wang 22 , Reference Du, Gan and Li 25 ), we found that the significant increase in the CP population was accompanied by a sharp increase in the E. coli populations in the caecal content. This may be because the challenge increases mucus production of birds owing to intestinal inflammation, and the mucus serves as a growth substrate for some caecal bacteria( Reference M’Sadeq, Wu and Swick 40 ). Lactobacillus is the well-known beneficial gut bacteria, which is predominant in the ileam and the fourth largest groups in the caecum( Reference Gong, Si and Forster 44 ). In our study, the number of Lactobacillus bacteria in the ileum and caecum were not influenced following the EM/CP challenge. Similar results were observed in chickens infected with C. perfringens ( Reference Du, Gan and Li 25 , Reference Jia, Slominski and Bruce 42 ). However, some investigators reported that C. perfringens challenge significantly increased the Lactobacillus population in the intestinal digesta( Reference Liu, Guo and Wang 22 , Reference M’Sadeq, Wu and Swick 40 ). The inconsistent results might be related to the strain, dosage and frequency of C. perfringens infection, which need further study. As reviewed by Hardy et al. ( Reference Hardy, Alany and Russell 45 ), previous researches have reported the antimicrobial properties of l-arginine in humans and animals. To the best of our knowledge, there are no published studies on the effects of dietary arginine on the CP population in the guts of chickens. In this experiment, l-arginine supplementation reduced the CP population in the ileum and caecum of broiler chickens. One of the reasons for this result could be that l-arginine stimulates intestinal epithelial cell proliferation and survival via the regulation of the mammalian target of rapamycin( Reference Bauchart-Thevret, Cui and Wu 46 ). When the birds are infected with enteric pathogens, the restoration of normal epithelial cell turnover makes it challenging for the pathogens to maintain a stable niche for colonisation( Reference Ashida, Ogawa and Kim 47 ).
SIgA is the primary immunological barrier that prevents intraluminal pathogens from colonising to the intestinal mucosa, and maintains homoeostasis with the commensal microbiota( Reference Johansen and Kaetzel 48 ). The stimulation of mucosal IgA requires for large doses of bacteria, the extent of which depends on the total bacterial dose( Reference Papp, Sipeki and Vitalis 49 ). Our data illustrated that ileal sIgA levels increased with the enhanced colonisation of C. perfringens and E. coli in challenged birds 1 d after the challenge (day 21), which was corresponding with the results of previous studies( Reference Du, Wang and Gan 10 , Reference Sun, Liu and Guo 50 ). Thus, we speculated that in the early period of infection large amounts of sIgA were induced by the overgrowth of enteropathogenic bacteria to defend against acute intestinal injury. Polosukhin et al. ( Reference Polosukhin, Cates and Lawson 51 ) observed that parents with chronic obstructive pulmonary disease had reduced sIgA in bronchoalveolar lavage. He stated that bronchial epithelial structural abnormalities led to localised sIgA deficiency( Reference Polosukhin, Cates and Lawson 51 ). Another research also reported that caecal sIgA production was decreased in dextran sulphate Na-induced chronic colitis( Reference Zhao, Song and Deng 52 ). Thus, we considered the reduction of ileal sIgA levels in challenged birds 8 d after the challenge (day 28) as an intestinal epithelial impairment. The present study showed that sIgA levels were increased by l-arginine supplementation in the jejunum on day 28, leading to protection of the intestinal mucosal surface( Reference Viana, dos Santos and Generoso 16 ).
Pattern recognition receptors (PRR) detect various microbial infections and trigger inflammation via specific signalling pathways to defend against invading pathogens. TLR and NOD-like receptors (NLR) are two major PRR families( Reference Kawai and Akira 53 ). In the current experiment, we analysed the mRNA expression levels of intestinal TLR2 and NOD1. TLR2 can detect peptidoglycan and lipoteichoic acid from gram-positive bacteria on the cell surface( Reference Kawai and Akira 53 ). Our data showed that EM/CP challenge led to a higher mRNA expression of TLR2 in the jejunum of broilers. Similar result was reported by Du et al. ( Reference Du, Wang and Gan 10 ). NOD1 and NOD2 are well-known members of the NLR family, which recognise bacterial peptidoglycan in the cytoplasm in mammals( Reference Kawai and Akira 53 ). As the nucleotide sequence of NOD2 in chickens has not been uncovered yet, we only determined the changes of NOD1 here. In the present study, EM/CP co-infection significantly increased the intestinal NOD1 gene expression. Guo et al. ( Reference Guo, Li and Liu 54 ) reported that CP could be recognised by NOD1 in chicken primary intestinal epithelial cells. NOD1 −/− mice infected with Chlamydophila pneumoniae exhibited delayed bacterial clearance and delayed neutrophil recruitment with evidence for defective NO and blunted cytokine production( Reference Shimada, Chen and Dempsey 55 ). In our study, the elevated intestinal mRNA expression of NOD1 with l-arginine supplementation indicated that l-arginine promoted the recognition of invasive pathogenic components in the cytoplasm. However, how CP peptidoglycan is transferred to the cytosol to combine with NOD1 and how l-arginine stimulates NOD1 remain unclear.
To clarify the effects of l-arginine on gut pro- and anti-inflammatory responses in the NE model, we examined the IFN- γ and IL-10 mRNA expression. Our results showed that addition of l-arginine elevated the mRNA expression levels of both IFN- γ and IL-10 in the small intestine. Shang et al. ( Reference Shang, Hsu and Yeh 56 ) suggested that l-arginine increased the gene transcripts of both Th1 (IL-2 and IFN- γ) and Th2 (IL-4 and IL-10) cytokines in splenocytes of rats with sepsis induced by caecal ligation and puncture. Viana et al. ( Reference Viana, dos Santos and Generoso 16 ) obtained similar results in the serum of mice with intestinal obstruction. Previous studies have demonstrated that IFN-γ evokes epithelial apoptosis and impairs intestinal barrier function( Reference Yang, Fan and Teitelbaum 57 , Reference Yang, Yu and Sun 58 ). However, Kominsky et al. ( Reference Kominsky, Keely and MacManus 59 ) found that IFN-γ up-regulates cellular methylation pathways that play a protective role in intestinal epithelium. More recently, Kominsky et al. ( Reference Kominsky, Campbell and Ehrentraut 60 ) demonstrated that IFN-γ specifically increased IL-10 receptor 1 (IL-10R1) expression, as well as up-regulating IL-10 gene expression in the intestinal epithelium. Knockdown of IL-10R1 led to increased intestinal permeability in mice. This discrepancy may be due to the differences in challenge model and experimental animals. IFN-γ secretion contributes to macrophage activation, improvement in microbicidal activity and enhanced expression of the epithelial receptor for the sIgA( Reference Sollid, Kvale and Brandtzaeg 61 , Reference Fabri, Stenger and Shin 62 ). In addition, IL-10 has been previously reported to stimulate the production and secretion of sIgA( Reference Briere, Bridon and Chevet 63 ). Thus, our data showed that l-arginine elevated the mRNA expression of IFN- γ and IL-10, which might contribute to the clearance of pathogens and improvement of intestinal integrity( Reference Viana, dos Santos and Generoso 16 ).
NO has been reported to inhibit the growth of Salmonella species, Actinobacillus species, Streptococcus pyogenes and Staphylococcus aureus ( Reference Alam, Akaike and Okamoto 64 , Reference Ulett and Adderson 65 ), but it does not affect certain bacterial strains such as group B streptococci( Reference Ulett and Adderson 65 ). Thus far, data regarding the bacteriostasis effect of NO against CP in vitro are scarce. In our experiment, culturing CP with over 2000 μm SNAP significantly retarded its growth, indicating that NO exerts direct antimicrobial action against CP. l-Arginine has been considered as the sole NO source in the host, and supplementation with l-arginine has been shown to increase the NO levels in both animals and cells( Reference Khajali, Tahmasebi and Hassanpour 66 , Reference Stadelmann, Hanevik and Andersson 67 ). Viana et al. ( Reference Viana, dos Santos and Generoso 16 ) confirmed that the role of arginine in reducing bacterial translocation and stimulating cytokines and sIgA in mice after intestinal obstruction were mediated by NO production. In our study, arginine treatment reduced CP populations, and improved intestinal permeability might be related to NO production.
In conclusion, l-arginine maintained intestinal integrity and accelerated pathogen clearance in broiler chickens, which might at least partly be associated with accelerated pathogen recognition, modulation of gut pro-inflammatory and anti-inflammatory cytokines, enhanced mRNA expression of TJ proteins and sIgA and NO production. In our study, diets including 1·87 % l-arginine fed for 28 days could alleviate intestinal injury of broiler chickens with NE.
Acknowledgements
The authors thank Weiwei Wang, Zhui Li, Yunshuang Yue, Hao Fan and Liping Gan for their assistance in this study.
This work was financially supported by the Modern China Agricultural Research System Program (CARS-42-G13).
B. Z. carried out the animal experiment, performed sample analysis and wrote the manuscript. Z. L. assisted with sample collection and data analysis. H. L. helped animal feeding and sample collection. S. G. and D. L. gave advise to the manuscript writing. Y. G. contributed to the experimental design.
The authors declare that there are no conflicts of interest.















