Tea, brewed from leaves of the leaves of the Camellia Sinensis bush, is the most popular beverage worldwide, after water. Tea may be categorised as black, green or oolong- depending the method of production. Thought to have been consumed since 2700 BC, tea has a long cultural history of being thought of as health drink(Reference Weisburger1). Prostate cancer has the highest incidence of all cancers in men and is a prominent cause of cancer mortality(Reference Siegel, Miller and Jemal2). There is considerable interest in exploring the impact of the widely consumed beverage tea, on men’s risk of this common cancer.
Research interest in the potential anticancer benefits of tea centres on its rich content of flavonoid polyphenols, a plant-derived micronutrient group(Reference Weisburger1). The notable flavonoid polyphenols in tea catechin, epicatechin, epigallocatechin-3-gallate and proanthocyanidins(Reference Higdon and Frei3–Reference Liao, Yang and Park5). The flavonoids in tea have been proposed to have anticancer benefits(Reference Williams, Spencer and Rice-Evans6). Whilst flavonoids do possess some antioxidant activity, this is not thought to be significant in vivo in the context of other more potent redox influencers(Reference Williams, Spencer and Rice-Evans6). The potential anticancer properties of tea are instead thought to include modulation of cell signalling pathways for oncogenic transformation, inflammation, apoptosis and angiogenesis(Reference Liao, Yang and Park5,Reference Pietinen, Malila and Virtanen7,Reference Voorrips, Goldbohm and van Poppel8) .
Clinical intervention studies in patients with a range of different cancers have involved small sample groups but have demonstrated good tolerability in some indication of possible clinical benefit. A phase II trial in forty-two patients from the Mayo Clinic administered a green tea extract to patients with previously untreated chronic lymphocytic leukaemia(Reference Shanafelt, Call and Zent9). In this single-arm study, fifteen patients experienced a > 20 % reduction in lymphocyte count. Additionally, eleven out of twelve patients with palpable lymphadenopathy had at least a 50 % reduction in palpable lymph node volume. There have also been a few studies examining green tea supplementation in established prostate cancer. A phase II single-arm study of twenty men administered green tea extract in the median 34-d interval between diagnosis with localised prostate cancer and radical prostatectomy(Reference McLarty, Bigelow and Smith10). Despite the short treatment duration, there was a mean 10·4 % decrease in prostate-specific antigen (P = 0·012). The double blind UK National Cancer Research Network (NCRN) pomi-T randomised 203 men with localised prostate cancer 6 months of either placebo or a supplement containing green tea extract, as well as extracts from other polyphenol-rich foods (pomegranate, broccoli and turmeric)(Reference Thomas, Williams and Sharma11). Men in the treatment group experienced a significantly lower median prostate-specific antigen rise (difference 63·8 % ANCOVA, P = 0·0008).
In light of this emerging evidence for possible clinical activity of green tea in prostate cancer, there is considerable interest in whether there is a role for the prevention of prostate cancer. A recently updated Cochrane review conducted a meta-analysis of clinical intervention studies of green tea supplementation for the prevention of prostate cancer(Reference Filippini, Malavolti and Borrelli12). Three studies, small preventative benefit were reported but the conclusion was this was of low certainty, due to the small sample sizes. These studies also included men with a high risk of prostate cancer due to existing pre-malignant histological abnormalities. In our dataset, men did not have pre-existing risk factors so is a better representation of the general population.
Epidemiological studies have linked a reduced risk of breast and prostate cancer with long-term tea intake, although the results are not all consistent(Reference Siegel, Miller and Jemal2,Reference Thomas, Williams and Sharma11,Reference Lambert and Yang13) . The 2006 Ohsaki prospective cohort study of 40 530 people in Japan reported that green tea consumption reduced all-cause mortality but found no association with cancer mortality(Reference Kuriyama, Shimazu and Ohmori14). The 2020 Cochrane review of epidemiological studies linking green tea consumption with cancer risk concluded that that there is insufficient evidence for a benefit or risk(Reference Filippini, Malavolti and Borrelli12). The review also examined prostate cancer risk in relation to green tea intake, encompassing thirteen studies with 127 239 participants. The finding was of a reduced relative risk, but the authors acknowledged the further confirmatory research was needed because that despite the large effect size, there were inconsistencies that rendered this result very low confidence. For these reasons, together with the popularity of tea drinking and high incidence of prostate cancer, it was deemed necessary to conduct this new analysis specifically looking at tea intake.
The Prostate, Lung, Colorectal and Ovarian (PLCO) Trial was a large-scale randomised control trial of screening by the US National Cancer Institute (NCI)(Reference Zhu, Pinsky and Kramer15). PLCO datasets, which include dietary data, have been a rich resource for epidemiological studies(Reference Reger, Zollinger and Liu16,Reference Bhagwat, Haytowitz and Holden17) .
Methods
We analysed data from 25 097 men out of 49 104 men and women enrolled to the intervention arm of the intervention arm of the 155 000 participant PLCO screening trial who were recruited from ten screening centres across the USA between November 1993 and July 2001.
Baseline characteristics were determined for the men in intervention cohort (n 25 097) (Table 1). For the analysis concerned with the relationship between tea consumption and prostate cancer incidence, subjects were excluded if did not complete the baseline questionnaire, were not asked to complete the Dietary Questionnaire (DQX) at baseline, completed the DQX but determined to be invalid, were not followed up after the enrolment or had cancer prior to enrolment in the PLCO study.
Table 1. Baseline characteristics of study participants split into tertiles (T) according to dietary intake of tea (g/d) during the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO)
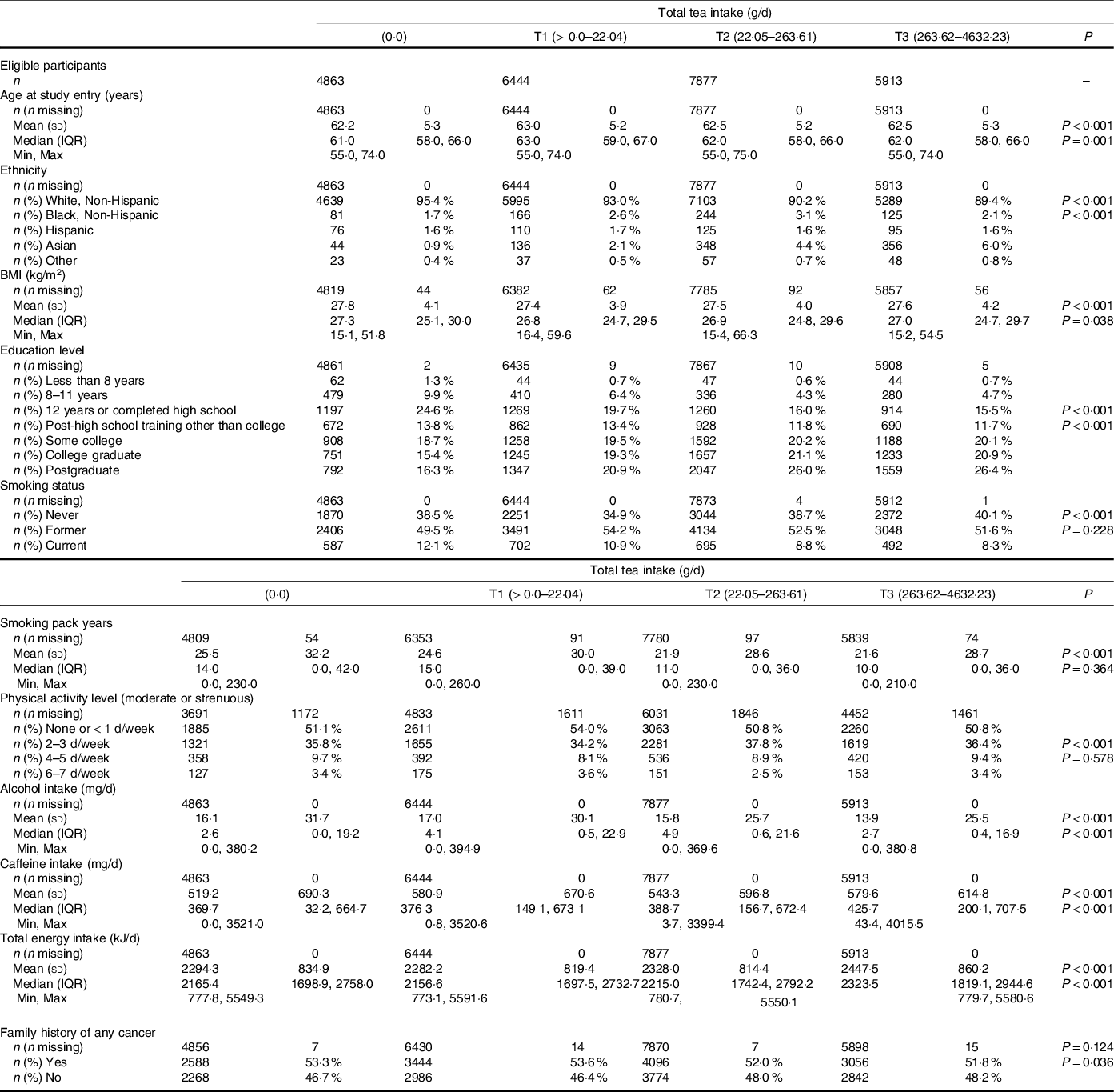
Histologically confirmed cases of prostate cancer were reported in 3088 men (12·3 %) during a median 11·5-year follow-up. Tea consumption was assessed with a dietary questionnaire which assessed food frequency and was completed at the time of randomisation of the intervention arm. Men were split into tertiles (T) according to dietary intake of tea drinks assessed by weight in g/d. Daily tea consumption in T1, T2 and T3 was estimated to be 1·81–21·74 g, 22·05–219·2 g and 258·2–4632·23 g, respectively. The FFQ is a 137-item FFQ developed to assess usual diet consumption during the past year. Dietary intake of energy and nutrients was calculated by multiplying the amount of energy and nutrients in a standard portion size of each food item by the reported frequency of consumption. The questions related to tea drinking included hot tea, iced tea and whether decaffeinated or caffeinated with ten categories of intake.
The vast majority (96·8 %) of the PLCO participants completed the baseline questionnaire that solicited information on age, ethnicity, BMI (kg/m2), education level, physical activity, cigarette smoking, family history of prostate and other cancers, vegetable and total energy intake.
Men in the intervention arm were offered annual prostate-specific antigen blood test and digital rectal exam for screening prostate cancer during their first 6 years of participation in the trial and follow-up continued for at least 7 additional years. Any diagnosis of cancer was reported in the clinical research folder whether detected by screening in the first 6 years or clinically for the next 7 years. In this follow-up period, men were referred for diagnostic evaluation including a prostate biopsy, if they had a prostate-specific antigen test > 4 ng/ml or if they had nodularity, induration, asymmetry or a loss of anatomic landmarks of the prostate on digital rectal examination. During a median follow-up of 11·5 years, 3088 cases of any grade of prostate cancer were identified from the 25 097 eligible men.
Statistical analysis
Demographic, anthropometric and lifestyle characteristics of subjects were compared across tertiles of total tea intake and the no tea intake group using the χ 2 test or Fisher’s exact test for categorical variables and the Kruskal–Wallis test for continuous variables. Monotonic trend (upward or downward) across groups was assessed using linear regression for continuous variables and the Cochran–Armitage test for categorical variables. Between-group baseline differences were also examined between subjects who developed any prostate cancer and those who were free from this malignancy during the specified follow-up period, using the χ 2 test or Fisher’s exact test for categorical variables and the Student’s t-test or Wilcoxon Mann–Whitney test for continuous variables.
Cox proportional hazards regression models were used to assess the association between tea intake and prostate cancer incidence, both unadjusted and sequentially adjusting for demographics, history of cancer, lifestyle and diet characteristics that were shown to be significantly different between tea intake groups. Hazard ratios, 95 % CI and P-values were presented for each tea intake group (including the non-tea drinkers group) compared against the reference group (no intake T = 0, with the other categories of tea intake (Table 2). Among tea drinkers, the lowest tertile (T = 1) was compared with the highest tea intake tertial (T3) (Table 3). All statistical analyses were performed using R Version 3.6.2.
Table 2. Survival analysis results for the incidence of prostate cancer diagnosis across range of tea intake (g/d). The categorical cox regression model presents the hazard ratio (HR), 95 % CI and corresponding P-value for lowest tertile tea intake group with the reference group comprising those consuming the highest level of tea (T3 = 258·2–4632·23 g tea/d). The overall P-value presents the overall level of association between tea intake and the lower incidence of prostate cancer diagnosis
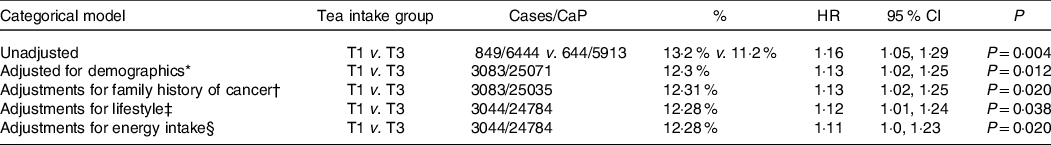
HR, hazard ratio.
* Age, sex, race and education level.
† Family history of cancer.
‡ Smoking, BMI, physical activity levels, caffeine and alcohol consumption.
§ Energy intake.
T1 = 1.81–21.7423 g tea/d.
T3 = 258.2–4632.23 g tea/d.
Table 3. Survival analysis results for the incidence of prostate cancer diagnosis across range of tea intake (g/d). The categorical cox regression model presents the hazard ratio for each tertile group of tea intake with the reference group comprising those consuming 0 g tea/d. The overall P-value presents the overall level of association between tea intake and the lower incidence of prostate cancer diagnosis
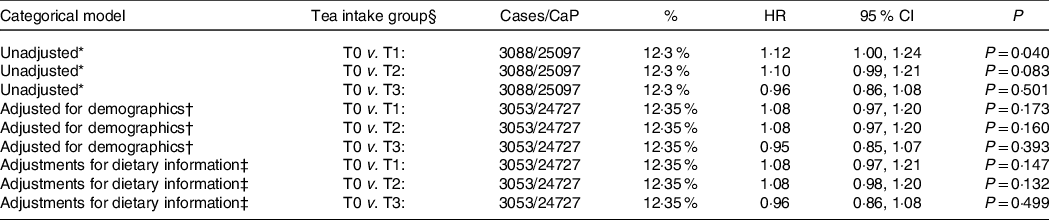
HR, hazard ratio.
* Unadjusted model.
† Model adjusted for demographics – age, sex, race, smoking status, cigarette pack-years, education and family history of cancer.
‡ Model further adjusted for dietary information – alcohol use, energy (kJ) intake, red meat intake, fruit intake and caffeine intake.
§ T1 = 1.81–21.7423 g tea/d, T2 = 22.05–219.2 g tea/d, T3 = 258.2–4632.23 g tea/d).
Results
The baseline characteristics (Table 1) showed there were statistically significant between-group differences (P < 0·001) found in age, ethnicity, BMI, smoking, exercise levels, educational level, alcohol consumption and energy intake according to level of tea intake. Higher tertile tea drinkers, were more likely to be of Asian ethnicity, have a higher overall energy intake, lower levels of alcohol consumption and were more likely to have never smoked. As expected, total caffeine intake was higher among those in the upper tertile of tea drinkers. No significant difference was found in family history of cancer between groups. These factors were taken into account in the adjusted survival analysis.
Of the 4863 men who never drank tea, 565 (11·6 %) developed prostate cancer (CaP) and of the 5913 men who were in the upper tertile of tea intake 664 (11·2 %) developed CaP. Using the Cox proportional hazards regression models, men who never drank tea had no greater risk of prostate cancer to men in the upper tertile of tea intake (11·6 % v. 11·2 % hazard ratio 0·96, sd 0·86–1·08, P = 0·501) (Table 2). Likewise there was no statistical difference, after adjustment for demographics and lifestyle between non-tea drinkers, low and moderate intake tea drinkers (T1 and T2 groups), see Table 3.
Of the 20 234 men who drank tea, 849 of 6444 (13·2 %) developed CaP in the lower tertile of tea drinkers and 664 of 5913 (11·2 %) in the upper tertile of tea intake. Using the Cox proportional hazards regression models, there was a statistically significant difference of 13·2 % v. 11·2 % hazard ratio 1·16; (95 % CI 1·05, 1·29), P = 0·004). This pattern persisted with adjustments for demographics of age, race and education level (T1 v. T3, P = 0·012); family history of cancer (P = 0·020); lifestyle habits of smoking, BMI, caffeine and alcohol consumption (P = 0·038); and other dietary factors of energetic intake (P = 0·020), see Table 3.
Conclusions
In this large prospective cohort study, we found that there was no difference in the risk of prostate cancer between men who did not drink tea and those who drank the low, moderate or highest quartile of tea intake. The data suggest that tea drinkers can be reassured that they can continue to enjoy this popular beverage without increasing their risk of CaP. Among tea drinkers, there was a significant inverse association of tea consumption with the risk of prostate cancer. These associations were independent of established or suspected risk factors for prostate cancer and differences in baseline characteristics. The effect of tea consumption among the lowest and highest consumers of tea drinkers was, however, small (mean 2 % difference), so although statistically significant, because of the large numbers in this dataset, it is debatable whether this is clinically relevant. Also, as the dataset only specifically screened for CaP in the first 6 years of the study for those who presented clinically thereafter, a potential of detection bias cannot be excluded.
Another potential weakness of this study is that, the DQX did not differentiate between black tea and green tea, or ask about the strength of the tea or the brands used, all of these variables can significantly affect the levels of these polyphenols. Tea brands can also vary in the age of the leaves taken from the bush, with the uppermost, younger tips being richest in polyphenols(Reference Chen, Liang and Lai18). Moreover, according to the USDA Database for the Flavonoid Content of Selected Foods, brewed tea can deliver 10 times more than bottled green tea(Reference Bhagwat, Haytowitz and Holden17). With this variability in mind, it is difficult to estimate the quantity of polyphenols within each group in this dataset. In terms of quantity of tea cups consumed, based on a g:ml conversion of 1:1, the lowest tertial would be approximately one cup of brewed tea 2–4 d a week and for the upper tertial 4–10 large cups a day or up to 8 pitchers of ice tea.
A further potential limitation of this present study is that dietary intake of tea was estimated using a FFQ at baseline, and dietary habits could have changed the subsequent years of follow-up. It is also well known that recall errors often occur in questionnaire-based dietary assessment. If non-differential, could result in an attenuation of the strength of the associations of interest. What’s more, the bioavailability of ingested polyphenols can be influenced by host factors such as bacterial microflora in the gut(Reference Corrêa, Rogero and Hassimotto19) and the combination with other polyphenol-rich foods such as turmeric, broccoli and pomegranate which is known to have significant synergistic influence on biological processes which could affect cancer initiation and progression(Reference Thomas, Williams and Sharma11,Reference Parada and Aguilera20,Reference Niedzwiecki, Roomi and Kalinovsky21) .
These data add weight to the discussion concerning tea consumption and prostate cancer but is by no means conclusive. Considering these factors, these data do not suggest that starting to drink tea, among previous non-tea drinkers would be useful preventative strategy against CaP. Moving forward further prospective intervention studies are required to ascertain whether higher tea take could have a role in prostate cancer incidence. Clearly, any dietary interventions should consider healthy lifestyle along with fruit, vegetables, fibres and phytochemical-rich foods(Reference Kirsh, Peters and Mayne22–Reference Giovannucci, Rimm and Liu24). In view of the uncertainties of polyphenol content in tea as a beverage, concentrated tea, in supplement form, may aid the design, standardisation and control of a randomised trial. Likewise, it would be prudent to investigate the combination of tea with other polyphenol-rich whole foods bearing in mind, results from previous intervention studies. Our trial group is planning a preventative study in higher risk men in the near future.
Acknowledgements
The authors thank the National Cancer Institute (NCI) for access to its data collected from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. The statements contained herein are solely those of the authors and do not necessarily represent or imply concurrence or endorsement by NCI.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
R. T. directed and devised the study and prepared the draft manuscript. B. G. contributed to interpreting results, updated results tables, contributed a literature search and prepared the final manuscript. A. M.: main statistical analyser. B. S.: head of statistical analysis and overall lead. M. W.: administrative head of the study – data co-ordination and registration.
The authors declare no conflicts of interests.








