Osteoporosis is a chronic disease characterised by low bone strength, predisposing to an increased risk of fracture( 1 ). Postmenopausal women have a higher risk of developing osteoporosis because of declining oestrogen concentrations associated with menopause( Reference Masse, Dosy and Tranchant 2 ). One treatment for osteoporosis is hormone replacement therapy; however, its use can result in adverse effects, such as the induction of hormone-dependent breast and uterine cancers( Reference Beral, Bull and Reeves 3 ).
Epidemiological studies indicate that women with high soya intake have a lower risk for osteoporosis compared with those consuming a typical Western diet( Reference Messina 4 ). In addition, many studies indicate that soya isoflavone (ISO)-rich extracts have bone-preserving functions in postmenopausal women( Reference Wu, Oka and Tabata 5 , Reference Taku, Melby and Takebayashi 6 ) and oestrogen-deficient animals( Reference Ishimi, Arai and Wang 7 ). Soyabean ISO are structurally similar to oestrogen and bind to oestrogen receptors( Reference Schmitt, Dekant and Stopper 8 ), suggesting that they exhibit weak oestrogenic action in various tissues and therefore might prevent postmenopausal disorders such as osteoporosis( Reference Taku, Melby and Takebayashi 6 ). However, the results of randomised-controlled trials on the bone-protective effects of soya ISO in menopausal women are controversial. Some studies have shown that ISO supplements have a modest effect on bone-sparing or on bone metabolism markers( Reference Taku, Melby and Takebayashi 6 , Reference Wu, Oka and Ezaki 9 , Reference Marini, Minutoli and Polito 10 ), whereas others considering ISO supplementation with simultaneous Ca and vitamin D supplementation have reported no effects( Reference Wong, Lewis and Steinberg 11 – Reference Tai, Tsai and Tu 13 ).
Equol is a metabolite of the ISO daidzein, and it is produced by bacterial microbiota in the distal intestine and colon. It possesses a stronger affinity for oestrogen receptors and induces transcription more strongly compared with other soya ISO( Reference Morito, Hirose and Kinjo 14 ). Equol has been shown to prevent bone loss in postmenopausal women( Reference Tousen, Ezaki and Fujii 15 ) and oestrogen-deficient animals( Reference Fujioka, Uehara and Wu 16 ). The clinical effectiveness of soya ISO is proposed to occur following the induction of equol production in the intestine( Reference Lampe 17 ). Conversely, Pawlowski et al. ( Reference Pawlowski, Martin and McCabe 18 ) recently reported that soya ISO are effective bone-preserving agents in postmenopausal women regardless of their equol-producing status. Intestinal bacteria have an essential role in daidzein metabolism, and specific equol-producing bacteria are required( Reference Atkinson, Frankenfeld and Lampe 19 ). Most studies suggest that animals, but not all humans, can produce equol( Reference Wu, Oka and Ezaki 9 , Reference Tousen, Abe and Ishida 20 , Reference Tousen, Uehara and Kruger 21 ). At least 50–60 % of the Asian population can metabolise daidzein to equol( Reference Akaza, Miyanaga and Takashima 22 ), which is remarkably higher than the reported 25–30 % in Western countries( Reference Setchell and Cole 23 ). The reasons for these differences are unclear, but habitual dietary patterns may influence the metabolism of ISO and the production of equol( Reference Lampe, Karr and Hutchins 24 ). Lampe et al. ( Reference Lampe, Karr and Hutchins 24 ) reported that diets of equol producers are richer in dietary fibres and carbohydrates compared with those of non-producers. Therefore, several studies have examined the effect of both prebiotics and probiotics on equol production in an attempt to establish the beneficial effects of ISO( Reference Lampe, Karr and Hutchins 24 ). Moreover, it is reported that daidzein is metabolised in the colon to equol by bacterial species such as Bifidobacterium spp. and Lactobacillus spp.( Reference Xu, Harris and Wang 25 – Reference Tsangalis, Ashton and McGill 27 ). However, the in vivo metabolic processes of these intestinal microbiota remain to be fully elucidated.
Resistant starch (RS) is a type of dietary fibre that includes all starch and starch degradation products that are not absorbed in the small intestine of healthy humans( Reference Topping, Fukushima and Bird 28 ). RS is fermented to a large extent by microbiota in the colon, resulting in the production of SCFA, which lowers the pH in the colon( Reference Topping and Clifton 29 ). Several studies have characterised the potential of RS to induce alterations in the composition of the gut microbiota and have reported increases in Bifidobacteria, Lactobacillus spp. and Bacteroides ( Reference Tousen, Abe and Ishida 20 , Reference Zhang, Wang and Zheng 30 – Reference Wang, Brown and Khaled 32 ).
Oestrogen deficiency results in bone loss associated with altered immune status. The gut microbiota modulates the host metabolism and development of the immune status. Recent studies have suggested an important role for gut–bone signalling pathways and microbiota in regulating bone health via the modification of immune status( Reference Sjogren, Engdahl and Henning 33 – Reference Britton, Irwin and Quach 35 ). Ohlsson et al. ( Reference Ohlsson, Engdahl and Fak 34 ) reported that Lactobacillus spp. treatments reduced the expression of inflammatory cytokines in bone, resulting in attenuated bone resorption in ovariectomised (OVX) mice. However, the mechanism by which the gut and the intestinal microbiota regulate bone density is unknown.
We previously reported that equol treatment in OVX mice prevented bone loss and altered bone marrow cell gene expression of the inflammatory indices induced by oestrogen deficiency( Reference Nishide, Tadaishi and Kobori 36 ). Therefore, we hypothesised that the intake of a combination of ISO and RS might modulate intestinal microbiota, promote equol production and/or regulate bone inflammation, resulting in cooperative effects on suppressed bone loss caused by oestrogen deficiency. In this study, we examined the combined effects of diet supplemented with ISO and RS on these events using an osteoporotic animal model.
Methods
Animals and diet
Female ddY strain mice, aged 8 weeks, were purchased from the Shizuoka Laboratory Animal Center (Shizuoka, Japan). The mice were housed in individual cages in a temperature- and humidity-controlled room (23±1°C and 60±5 % relative humidity) with a 12 h light–12 h dark cycle. The mice were given free access to an AIN-93G diet with maize oil instead of soyabean oil for 4 d before surgery was performed( Reference Reeves, Nielsen and Fahey 37 ). All ingredients for the AIN-93G diet were purchased from Oriental Yeast Co., Ltd. The mice were either sham operated (Sham, n 7) or underwent OVX on the same day. OVX mice were randomly divided into four groups (n 7 each): OVX control (OVX); OVX fed 0·05 % ISO-supplemented diet (OVX+ISO); OVX fed 9 % RS-supplemented diet (OVX+RS); and OVX fed 0·05 % ISO- and 9 % RS-supplemented diet (OVX+ISO+RS). The mice were pair-fed their respective diets for 42 d with free access to distilled water during this period. The ISO and RS concentrations were determined in our previous studies; 0·05 % ISO or 9 % RS diets slightly inhibited bone loss in OVX mice (unpublished results). The sample size calculation was performed before the start of the study, and it indicated that five mice for each group would show a difference of 6·2 mg/cm2 between femur bone mineral density (BMD) means, assuming a sd of 2·6 mg/cm2, with power of 0·80 and a two-sided type 1 error of 0·05. Seven mice in each group were therefore considered an appropriate sample size for the study.
Table 1 shows the composition of the experimental diets, which were prepared according to the AIN-93G formulation( Reference Reeves, Nielsen and Fahey 37 ). Maize oil was used to eliminate any contamination from ISO in soyabean oil. Dry powdered ISO and RS were added to the diet instead of sugar or maize starch, respectively. Supplemented ISO (ISO content: 94·4 %, J-OIL MILLS, Inc.) contained the purified ISO conjugates daidzin (55·8 %), glycitin (27·3 %), genistin (10·3 %) and others (1·1 %). Aglycones were present at a concentration of 58·8 mg/100 mg of conjugates. The ISO composition was determined by the HPLC method( Reference Kudou, Fleury and Welti 38 ). For the supplemented RS, acid-hydrolysed high-amylose maize starch (HAS) was included as 68 % of the dried weight (Amylofiber®; J-OIL MILLS, Inc.)( Reference Nagahata, Kobayashi and Goto 39 ). When preparing the diet, the RS content of acid-hydrolysed HAS was 60 % (wet convention weight). ISO and RS were kindly provided by J-OIL MILLS, Inc.
Table 1 Composition of the experimental diets (g/kg diet)Footnote *
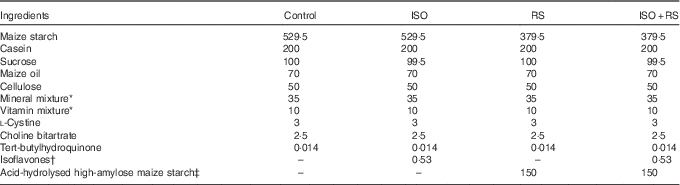
Control, control diet; ISO, isoflavones-supplemented diets; RS, resistant starch-supplemented diets; ISO+RS, isoflavone- and resistant starch-supplemented diet.
* Prepared according to the AIN-93G formulation( Reference Reeves, Nielsen and Fahey 37 ).
† The isoflavone conjugates (94·4 % isoflavones) were daidzin (55·8 %), glycitin (27·3 %), genistin (10·3 %) and others (1·1 %). Aglycones were present at a concentration of 58·8 mg/100 mg of conjugates.
‡ 68 % RS was included in acid-hydrolysed high-amylose maize starch (Amylofiber®; J-OIL MILLS, Inc.). When preparing the diet, the RS content of high-amylose maize starch was 60 % (the wet convention weight).
After 40 d of treatment, 48-h urine and faecal samples were collected and stored at −80°C until assayed. Mice were fasted overnight the day before anatomical investigations. After 42 d of treatment, the mice were euthanised by exsanguination under anaesthesia, weighed and then blood was collected in vacutainers and centrifuged at 7 g at 4°C for 15 min. The plasma was removed and stored at −80°C until it was assayed. The uterus was also removed and its wet weight was measured. The caecum was removed with its contents, weighed and stored at −80°C until it was assayed. The left femur was also removed to measure BMD, and the right tibia was removed to extract total RNA from the bone marrow cells.
All procedures involving animals were approved by the National Institute of Biomedical Innovation, Health and Nutrition Guidelines for the Care and Use of Laboratory Animals.
Radiographic analysis of the femur
BMD of the femur was measured by dual-energy X-ray absorptiometry (DEXA, model DCS-6-EX-R; Aloka) and calculated using the bone mineral content of the measured area. The scanned area of the mouse femur was divided into three equal parts: the proximal, midshaft and distal femur.
Analysis of trabecular microarchitecture by micro-computed tomography
The distal femurs were scanned at 48-μm intervals using a LaTheta experimental animal computed tomography system (Model LaTheta LCT-200; Hitachi Aloka Medical). Distal femur analyses were performed in a region of trabecular bone to the growth plate extending 1·4 mm towards the diaphysis excluding the outer cortical bone. The trabecular BMD, the ratio of trabecular area, the minimum moment of inertia of cross-sectional areas (MMICA) and the polar moment of inertia of cross-sectional areas (PMICA) were calculated using the LaTheta software (version 1.31; Hitachi Aloka Medical).
Caecal content weight, pH and β-glucosidase activity
Caecal contents were collected, caecal tissue was washed with saline and the weight of the contents was calculated by subtracting the tissue weight from the total weight. The pH of the caecal contents was measured with a pH meter (model B-212, Twin Compact pH meter; Horiba). β-Glucosidase activity was measured by determining the amount of p-nitrophenol from β-pyranoside. The reaction mixture contained 40 µl of substrate solution (50 mm-phosphate buffer at pH 7·0, 1 mm-p-nitrophenyl β-pyranoside) and 10 µl of sample solution at a 1:9 (v/v) dilution of the caecal sample in 50 mm-phosphate buffer at pH 7·0. The reaction mixture was incubated for 60 min at 37°C, and the p-nitrophenol concentration was measured spectrophotometrically at 405 nm after the addition of 200 µl of 0·1 m-NaOH. Enzyme activity was expressed as mol of p-nitrophenol per whole caecal content in 60 min.
Time-resolved fluoroimmunoassay for urinary daidzein, equol and genistein
Urinary daidzein, equol and genistein were analysed by the time-resolved fluoroimmunoassay method, as previously reported( Reference Brouwers, L'Homme and Al-Maharik 40 , Reference Wang, Lapcik and Hampl 41 ). Urine was hydrolysed by glucuronidase and sulphatase. Urinary daidzein, equol and genistein concentrations were determined by fluorescence using a DELFIA Victor 1420 multilabel counter (PerkinElmer). The final results were calculated as follows: concentration (read)×1/recovery×dilution factor (nmol/l)×urine 24-h volume.
DNA extraction from faeces
DNA was extracted from the faecal samples according to a method used by Nagashima et al. with modifications( Reference Nagashima, Mochizuki and Hisada 42 , Reference Nagashima, Hisada and Sato 43 ). The faecal samples were suspended in a solution containing 100 mm-TRIS-HCl (pH 9·0) and 40 mm-EDTA after being washed three times with sterile distilled water, and the faeces were then homogenised using a FastPrep FP100A Instrument (MP Biomedicals). DNA was extracted from the suspension using a GC series genomic DNA whole-blood kit and then purified using a Magtration 12GC system (Precision System Science).
PCR conditions and terminal restriction fragment length polymorphism analysis
Amplification of the faecal 16S ribosomal DNA (16S rDNA), restriction enzyme digestion, size fractionation of terminal restriction fragments and terminal restriction fragment length polymorphism data analysis were performed according to the method used by Nagashima et al. with modifications( Reference Nagashima, Mochizuki and Hisada 42 , Reference Nagashima, Hisada and Sato 43 ). Briefly, PCR was performed using total faecal DNA and primers for 5′-carboxy-fluorescein-labelled 516f and 1510r. The resulting 16S rDNA amplicons were treated for 3 h at 55°C with 10 U of Bs/I (5′-CCNNNNN|NNGG-3′) (New England Biolabs). The fluorescent-labelled terminal restriction fragments produced by digestion with Bs/I were analysed by electrophoresis on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems) in GeneScan mode (injection time was 30 s, and run time was 40 min).
RNA extraction from bone marrow of the tibia and quantitative real-time PCR
Total RNA was extracted from the bone marrow of the tibia using Isogen II (Nippon Gene) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesised from 1 μg of total RNA using PrimeScript RT Master Mix (Takara Bio). cDNA was quantified by real-time PCR using SYBR Premix Ex Taq II (Takara Bio). The PCR conditions were 95°C for 30 s, followed by forty cycles of 95°C for 5 s and 60°C for 30 s. The primer sequences used in PCR were as follows: β-actin forward, 5′-CCACAGCTGAGAGGGAAATC-3′; β-actin reverse, 5′-AAGGAAGGCTGGAAAAGAGC-3′; IL-7R forward, 5′-GCGGACGATCACTCCTTCTG-3′; IL-7R reverse, 5′-AGCCCCACATATTTGAAATTCCA-3′; CD-40-ligand (CD40L) forward, 5′-TCGGGAGCCTTCGAGTCA-3′; CD40L reverse, 5′-GATCCACTGCTGGGCTTCAG-3′( Reference Kong, Kim and Eom 44 – Reference Schroder, Vecchione and Jung 46 ). Results are expressed as the fold-change relative to the controls after normalising to β-actin gene expression levels.
Statistical analysis
Data are expressed as mean values with their standard errors. The differences in urinary equol, daidzein and genistein excretions and urinary equol:daidzein ratio between the OVX+ISO and OVX+ISO+RS groups were examined using the unpaired Student’s t test. The significance of differences in BMD, the trabecular area ratio and bone strength parameters of the femur were determined by ANCOVA and Fisher’s protected least significant difference test. Body weight was used as a covariate in the analysis of femoral BMD, trabecular BMD, the trabecular area ratio and bone strength parameters of the femur to adjust for possible confounding effects. The influences of ISO and/or RS treatment on the composition of faecal intestinal microbiota were evaluated by ANOVA and Tukey’s post hoc test when the data were normally distributed. When the data were not normally distributed and their variances were not equivalent, a non-parametric Kruskal–Wallis test was performed to determine significant differences between groups (P<0·05). The remaining data were analysed using one-way ANOVA. Differences between groups were assessed by Tukey’s post hoc test. Differences were considered significant when P<0·05. Statistical analysis was performed using the SPSS statistics version 19 software (IBM).
Results
Body and tissue weights
No statistically significant differences in initial and final body weights or total food intake were observed among groups (data not shown). The uterine weights of mice in the Sham, OVX, OVX+ISO, OVX+RS and OVX+ISO+RS groups were 84·0 (sem 12·6), 18·0 (sem 1·3), 22·3 (sem 1·2), 17·9 (sem 0·9) and 23·0 (sem 4·2) mg, respectively. Uterine weights from all OVX groups were significantly lower than those in the Sham group. Treatment with ISO, RS or a combination of ISO and RS did not affect uterine weight in OVX mice.
Caecal content, pH and β-glucosidase activity
Table 2 shows the caecal content, pH and β-glucosidase activity of OVX and Sham mice. Wet weight of the caecal contents and caecal β-glucosidase activity were significantly higher in the OVX+RS and OVX+ISO+RS groups compared with the Sham, OVX and OVX+ISO groups. Caecal pH was significantly lower in the OVX+RS and OVX+ISO+RS groups compared with the OVX and OVX+ISO groups. These results clearly indicated that caecal content, pH and β-glucosidase activity were affected by RS intake.
Table 2 Wet weights of the caecal content, pH and β-glucosidase activity in miceFootnote * (Mean values with their standard errors; n 7)
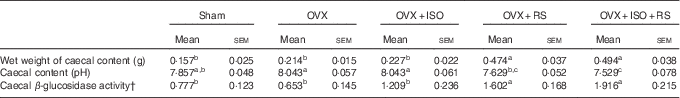
Sham, sham-operated mice fed the control diet; OVX, ovariectomised mice fed the control diet; OVX+ISO, OVX mice fed a 0·05 % isoflavone (ISO)-supplemented diet; OVX+RS, OVX mice fed a 10 % resistant starch (RS)-supplemented diet; OVX+ISO+RS, OVX mice fed a combination of 0·05 % ISO and 10 % RS-supplemented diets.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* Wet weight of caecal content, caecal content pH and caecal β-glucosidase activity were analysed using one-way ANOVA. Differences between groups were assessed by Tukey’s post hoc test (P<0·05).
† Mol of p-nitro phenol/whole caecal content/60 min.
Caecal microbiota analysis
Human intestinal micro-organisms predominantly consist of the members of approximately nine phylogenetic bacterial groups( Reference Nagashima, Mochizuki and Hisada 42 , Reference Nagashima, Hisada and Sato 43 ). The faecal microbiota was influenced by RS intake (Fig. 1). The ratios of Bifidobacterium spp. in the OVX+ISO+RS and OVX+RS groups were 10·29 (sem 2·46) % and 5·94 (sem 2·45) %, respectively. The abundance of Bifidobacterium spp. was significantly higher in the OVX+RS group (10·27 (sem 2·46) %) than in the Sham or OVX+ISO groups (0·27 (sem 0·12) % and 0·62 (sem 0·34) %, respectively) (P<0·05). However, there were no significant differences in the abundance of Bifidobacterium spp. among the other groups. Furthermore, the abundance of all other bacterial species among all groups was not significantly different.

Fig. 1 Composition of caecal intestinal microbiota in Sham mice and ovariectomised (OVX) mice fed either a control diet, an isoflavone (ISO)-supplemented diet (OVX+ISO), a resistant starch (RS)-supplemented diet (OVX+RS) or a combination of ISO and RS diets (OVX+ISO+RS) for 42 d. Values are means (n 7), with their standard errors represented by vertical bars. The influences of ISO and/or RS treatment on the composition of faecal intestinal microbiota were evaluated by ANOVA and Tukey’s post hoc test when the data were normally distributed. When the data were not normally distributed and their variances were not equivalent, a non-parametric Kruskal–Wallis test was performed to determine significant differences between groups (P<0·05). *,† Significantly different in the abundance of Bifidobacterium spp. between the Sham and OVX+RS groups, or between the OVX+ISO and OVX+RS groups (P<0·05), respectively. ![]() , Others;
, Others; ![]() , Clostridium cluster XVIII;
, Clostridium cluster XVIII; ![]() , Clostridium cluster XI;
, Clostridium cluster XI; ![]() , Clostridium subcluster XIVa;
, Clostridium subcluster XIVa; ![]() , Clostridium cluster IV; □, Prevotella;
, Clostridium cluster IV; □, Prevotella; ![]() , Bacteroides;
, Bacteroides; ![]() , Lactobacillales; ■, Bifidobacterium.
, Lactobacillales; ■, Bifidobacterium.
Urinary excretions of equol, daidzein and genistein
Urinary excretion of equol, daidzein and genistein was detected in mice treated with ISO, but not in the Sham, OVX and OVX+RS groups (Fig. 2). Urinary equol excretion was significantly higher in the OVX+ISO+RS group compared with the OVX+ISO group (Fig. 2(a)). The product–precursor relationship can be expressed as the excretion ratio of equol:daidzein in urine and is a reliable indicator of the amount of daidzein converted to equol. Although there was no significant difference in the urinary equol:daidzein ratio between the OVX+ISO and OVX+ISO+RS groups, the ratio in the OVX+RS+ISO group tended to be higher than in the OVX+ISO group (0·705 (sem 0·194) and 0·386 (sem 0·082), respectively) (P=0·0654) (Fig. 2(d)). No significant differences were observed in urinary daidzein and genistein excretion between the OVX+ISO and OVX+ISO+RS groups (Fig. 2(b) and (c)).

Fig. 2 Urinary excretion of equol, daidzein and genistein, and urinary equol:daidzein ratio in mice fed either an isoflavone (ISO)-supplemented diet (ovariectomised (OVX)+ISO) or a combination of ISO- and resistant starch-supplemented diets (OVX+ISO+RS) for 42 d. (a) Urinary equol excretion. (b) Urinary daidzein excretion. (c) Urinary genistein excretion. (d) Urinary equol:daidzein ratio. Values are means (n 7), with their standard errors represented by vertical bars. * Mean values were significantly different between the OVX+ISO and OVX+ISO+RS groups by unpaired Student’s t test (P<0·05).
Bone mineral density, trabecular bone mineral density and trabecular area ratio of the femur
The BMD of the mice femurs in each group are shown in Fig. 3. The BMD of whole, proximal, middle and distal regions of the femur in the OVX group were significantly lower than those in the Sham group (Fig. 3(A)–(D)). ISO treatment inhibited bone loss in the whole femur and proximal and distal regions of the femur (Fig. 3(A), (B) and (D)). Proximal femur BMD tended to be higher in the OVX+RS group than in the OVX group (Fig. 3(B)). Distal femur BMD was slightly higher in the OVX+ISO+RS group than in the OVX+ISO group; however, this difference was not statistically significant (Fig. 3(D)).

Fig. 3 Bone mineral density of femur was obtained from Sham mice and ovariectomised (OVX) mice fed either a control diet, an isoflavone (ISO)-supplemented diet (OVX+ISO), a resistant starch (RS)-supplemented diet (OVX+RS) or a combination of ISO- and RS-supplemented diets (OVX+ISO+RS) for 42 d. Bone mineral density (BMD) was measured by dual-energy x-ray absorptiometry analysis. (A) Whole femur BMD. (B) Proximal femur BMD. (C) Middle femur BMD. (D) Distal femur BMD. Values are means (n 7), with their standard errors represented by vertical bars. The significance of differences in BMD was determined by ANCOVA and Fisher’s protected least significant difference test. Body weight was used as a covariate in the analysis of femoral BMD to adjust for possible confounding effects. a,b,c,d Mean values with unlike letters were significantly different (P<0·05).
To confirm the effect of ISO and RS treatment on distal bone in OVX mice, bone morphometric analysis was performed using micro-computed tomography in the distal femoral metaphysis (Fig. 4 and 5). The connection rods were well maintained in the Sham group (Fig. 4(a)). However, in the OVX group, many of the connecting rods were missing (Fig. 4(b)). Treatment with ISO and ISO+RS prevented trabecular bone loss in OVX mice (Fig. 4(c) and (e)), whereas treatment with RS slightly inhibited trabecular bone loss (Fig. 4(d)). Trabecular BMD and the trabecular area ratio of the distal femur in OVX mice were significantly lower than in Sham mice (Fig. 5(A) and (B)). Conversely, treatment with combined ISO and RS significantly inhibited trabecular bone loss in the distal femur of OVX mice (Fig. 5(A) and (B)). Treatment with ISO alone significantly inhibited the decreased trabecular area ratio. Distal femur MMICA and PMICA bone strength parameters were significantly lower in the OVX group compared with the Sham group (Fig. 5(C) and (D)). However, treatment with combined ISO and RS in OVX mice significantly inhibited the decrease in these parameters in the distal femur (Fig. 5(C) and (D)). On the other hand, these were no statistical differences between the OVX group and the OVX+ISO or the OVX+RS groups.

Fig. 4 Micro-computed tomography (μCT) scan of trabecular bone of distal femur was obtained from (a) Sham mice and (b) ovariectomised (OVX) mice fed either a control diet, (c) an isoflavone (ISO)-supplemented diet (OVX+ISO), (d) a resistant starch (RS)-supplemented diet (OVX+RS) or (e) a combination of ISO- and RS-supplemented diets (OVX+ISO+RS) for 42 d.

Fig. 5 Trabecular bone mineral density (BMD), the trabecular area ratio and bone strength parameters of the distal femur were obtained from Sham mice and ovariectomised (OVX) mice fed either a control diet, an isoflavone (ISO)-supplemented diet (OVX+ISO), a resistant starch (RS)-supplemented diet (OVX+RS) or a combination of ISO and RS-supplemented diets (OVX+ISO+RS) for 42 d. Trabecular BMD, the trabecular area ratio and bone strength parameters were measured by micro-computed tomography scanning. (A) Trabecular BMD. (B) The trabecular area ratio. (C) Minimum moment of inertia of cross-sectional areas (MMICA). (D) Polar moment of inertia of cross-sectional areas (PMICA). Values are means (n 7), with their standard errors represented by vertical bars. The significance of differences in trabecular BMD, the trabecular area ratio and bone strength parameters of the femur were determined by ANCOVA and Fisher’s protected least significant difference test. Body weight was used as a covariate in the analysis of trabecular BMD, the trabecular area ratio and bone strength parameters of the femur to adjust for possible confounding effects. a,b,c,d Mean values with unlike letters were significantly different (P<0·05).
Expression of inflammation-related genes in bone marrow
Fig. 6 shows the expression of inflammation-related genes in the bone marrow of the mice. mRNA expression of IL-7R was significantly increased in OVX mice compared with Sham mice, and treatment with ISO and combined ISO+RS significantly reduced IL-7R gene expression (Fig. 6(A)). Moreover, mRNA expression of IL-7R in the OVX+ISO+RS group tended to be lower than that in the OVX+ISO group. Although differences among groups were not significant, expression of the CD40L gene tended to be lower in the OVX+ISO and OVX+ISO+RS groups compared with the other groups (Fig. 6(B)).

Fig. 6 mRNA expression of bone marrow cells collecting from the tibia was obtained from Sham mice and OVX mice fed either a control diet, an isoflavone (ISO)-supplemented diet (OVX+ISO), a resistant starch (RS)-supplemented diet (OVX+RS) or a combination of ISO- and RS-supplemented diets (OVX+ISO+RS) for 42 d. Expression levels of IL-7R and CD-40-ligand (CD40L) were determined by quantitative real-time PCR. The ordinate axis indicates the relative amount of mRNA compared with sham mice. Gene expression levels were normalised with β-actin. (A) IL-7R. (B) CD-40-ligand (CD40L). Values are means (n 7), with their standard errors represented by vertical bars. mRNA expression of bone marrow cells collecting from the tibia was analysed using one-way ANOVA. Differences between groups were assessed by Tukey’s post hoc test (P<0·05). a,b,c Mean values with unlike letters were significantly different (P<0·05).
Discussion
In the present study, we examined whether a diet supplemented with ISO and RS could modulate intestinal microbiota, promote equol production and/or regulate bone inflammation, resulting in the suppression of bone loss caused by oestrogen deficiency in mice. The combination of ISO and RS prevented an ovariectomy-induced decrease in trabecular bone mass and bone strength in the distal femur by modulating the enteric environment, including an increase in the abundance of Bifidobacterium spp. and equol production in the intestine, and regulating inflammation-related genes in the bone marrow.
The ability to produce equol depends on the presence of certain intestinal microbiota, indicating that the enteric environment can affect equol production( Reference Yuan, Wang and Liu 47 ). Our results showed that caecal contents were increased and pH was decreased in mice fed RS and the combination of ISO and RS compared with the control and ISO-alone diet groups. Moreover, there was an increase in β-glucosidase activity in the caecal contents and increased equol production in mice fed RS and the combination of ISO and RS. These data are consistent with our previous reports of mice receiving a diet supplemented with a combination of ISO or daidzein and prebiotics, such as fructo-oligosaccharide, polydextrose and raffinose( Reference Tousen, Uehara and Kruger 21 , Reference Ohta, Uehara and Sakai 48 ). The activity of β-glucosidase in the caecal contents is an indicator of the activity of intestinal enzymes that hydrolyse the glycoside bond of ISO conjugates, which stimulates the intestinal absorption of ISO aglycones. These results suggest that RS enhances both the metabolism of ISO conjugates to aglycone and the metabolism of aglycone to equol from daidzein.
Certain Bifidobacterium and Lactobacillus species have been suggested to have an important role in the metabolism of daidzein to equol( Reference Xu, Harris and Wang 25 – Reference Tsangalis, Ashton and McGill 27 , Reference Yuan, Wang and Liu 47 ). The present study demonstrated that the diets containing 0·05 % ISO (containing 55·8 % daidzin as a conjugate) and 9 % RS increased equol production (Fig. 2) by increasing the abundance of Bifidobacterium spp. in the intestinal microbiota (Fig. 1). Previously, we reported that a diet containing 0·1 % daidzein and 12 % RS (HAS) promoted equol production and increased the abundance of Bifidobacterium spp. in the faecal microbiota of OVX mice. Categorisation of the types of RS is based on factors that explain their resistance to degradation in the gastrointestinal tract( Reference Englyst, Kingman and Cummings 49 ), and the functions of RS depend on their resistance to degradation( Reference Martínez, Kim and Duffy 50 , Reference Jacobasch, Dongowski and Schmiedl 51 ). In this study, we used hydrolysed-HAS as RS, which is increased in the crystalline regions and which demonstrates increased resistance to enzymatic digestion compared with HAS( Reference Nagahata, Kobayashi and Goto 39 ). Martínez et al. ( Reference Martínez, Kim and Duffy 50 ) reported that some types of RS have functional differences in their effects on human faecal microbiota composition, indicating that the chemical structure of RS determines its accessibility by groups of colonic bacteria. Thus, hydrolysed-HAS might be more efficient in metabolising equol from daidzein by stimulating Bifidobacterium spp. in the intestine, resulting in enhanced equol production.
In our study, mice fed a combination of daidzin and RS produced equol, indicating that some rodents are equol producers. However, it might not be easy to enhance equol production in humans, especially in equol non-producers. Some prebiotic supplementations with soya have shown no effect on the equol-producing capacity in humans( Reference Tousen, Uehara and Abe 52 , Reference Larkin, Price and Astheimer 53 ). However, further studies are needed to confirm whether RS promotes equol production in humans.
The BMD of the femur, as measured by DEXA, was significantly lower in OVX mice than in Sham mice. ISO alone and the combination of ISO and RS prevented OVX-induced bone loss. Although there was no significant difference between the ISO and ISO+RS groups for whole femur BMD, mice fed a combination of ISO and RS maintained their distal BMD more effectively than mice fed ISO alone (Fig. 3(D)). Moreover, trabecular BMD and the trabecular area ratio of the distal femur in the ISO+RS group were significantly higher than those in the OVX group. The specific sites that benefitted most had high amounts of trabecular bone, such as the distal femur. Previously, we reported that equol significantly inhibited the loss of trabecular bone volume in the femoral distal metaphysis, but there was no significant effect in the whole femur( Reference Nishide, Tadaishi and Kobori 36 ). This might be because of bone metabolism being faster in trabecular compared with cortical bone. These results indicated that the combination of ISO and RS might be more efficient than ISO alone for trabecular bone BMD in OVX mice. In addition, some part of the ISO effects on trabecular bone might be caused by equol.
Combined ISO and RS treatment significantly inhibited the decrease in bone strength index, including the MMICA and PMICA, in the distal femur of OVX mice (Fig. 5(A) and (B)). Bone quality is recognised to be as important as BMD for bone strength. S-equol is reported to exhibit an ameliorating effect on bone strength( Reference Kimira, Katsumata and Suzuki 54 – Reference Sehmisch, Erren and Kolios 56 ). Mathey et al. ( Reference Mathey, Mardon and Fokialakis 55 ) reported that bone strength is improved by equol consumption, but not by genistein or daidzein when administered alone. These results suggest that equol is more effective than ISO for the bone strength index in OVX mice.
The association between inflammation and bone loss is well established. Previous studies demonstrated that bone inflammation is increased with oestrogen deficiency and is linked to osteoclastogenesis, and therefore bone resorption is derived by cytokine-producing activated T cells( Reference Kong, Feige and Sarosi 57 – Reference Li, Tawfeek and Bedi 59 ). In this study, ISO and/or RS inhibited the increase in OVX-induced IL-7R mRNA expression (Fig. 6(A)). Moreover, although there were no significant differences among all groups, ISO and the combination with RS slightly decreased the expression of CD40L, a key surface ligand expressed on T cells (Fig. 6(B)). IL-7R and CD40L have a key role in bone resorption stimulated by oestrogen deficiency. IL-7 is a pleiotropic immune-regulatory protein predominantly produced by stromal cells and by cells at inflammatory sites. Weitzmann & Pacifici( Reference Weitzmann and Pacifici 60 ) demonstrated that the administration of IL-7 stimulated osteoclastogenesis through T cells. T cells dysregulate bone homoeostasis in OVX mice through CD40L-mediated cross-talk between themselves and stromal cells, which results in enhanced osteoclastogenesis and osteoblastogenesis( Reference Li, Tawfeek and Bedi 59 ). Our present results support previous studies in that a diet supplemented with equol for 2 weeks prevented an OVX-induced increase in IL-7R and CD40L in OVX mice( Reference Nishide, Tadaishi and Kobori 36 ). These findings suggest that ISO or its combination with RS supplement might alter the inflammation status in bone marrow, resulting in attenuated bone loss in OVX mice.
The consumption of RS changes the composition of the microbiota and promotes the microbial fermentative production of SCFA, which putatively reduce gastrointestinal inflammation. Recent studies suggested an important role for gut–bone signalling pathways and the microbiota in regulating bone health via the modification of immune status( Reference Sjogren, Engdahl and Henning 33 – Reference Britton, Irwin and Quach 35 ). Britton et al. ( Reference Britton, Irwin and Quach 35 ) indicated that one possible indirect mechanism by which probiotic treatment could affect the suppression of bone loss in OVX animals was by alteration of the immune response by changing intestinal microbial communities found in OVX animals. A limitation of the current study is the lack of direct mechanistic experiments assessing the effects between the intestinal microbiota and bone. However, we showed that RS altered the enteric environment, including the caecal pH, weight of caecal content and increased abundance of Bifidobacterium spp. in the intestine, which slightly decreased inflammation-related gene expression in the bone marrow, and slightly suppressed OVX-induced trabecular bone loss. These results raise the possibility that RS affects bone inflammation via SCFA and microbiota in the intestine. Further studies are needed to elucidate these relationships.
In conclusion, treatment with a combination of ISO and RS increased equol production and prevented the OVX-induced decline in trabecular BMD and bone strength parameters in the distal femur by modulating the enteric environment, including an increase in Bifidobacterium spp. and alterations in inflammation-related gene expression in the bone marrow. However, there were no significant differences in bone parameters between the ISO+RS and ISO-alone groups in OVX mice. Our findings suggest that the combination of ISO+RS might alter the microbiota in the intestine and immune status in the bone marrow, resulting in attenuated bone loss in OVX mice. Further studies are necessary to define the mechanism of action of ISO and RS on the intestinal microbiota and immune system and on bone metabolism in oestrogen deficiency.
Acknowledgements
The authors thank Dr Seiichi Kasaoka of Bunkyo University for his advice on our research.
The present study was supported by J-OIL MILLS, Inc.
The contributions of each author were as follows: Y. I., Y. T., I. K. and Y. N. designed the study; Y. T., Y. M., C. M. and Y. N. carried out the study; Y. T. and Y. M. analysed the data; Y. T. and Y. I. prepared the manuscript.
Y. N. and I. K. are employees of J-OIL MILLS, Inc. All other authors have no conflicts of interest.













