CVD continues to be the leading cause of mortality in the USA( Reference Mozaffarian, Benjamin and Go 1 ). Although CVD risk is attributable to numerous factors, findings from a meta-analysis indicate that the magnitude of postprandial hyperglycaemia (PPH) induces endothelial dysfunction( Reference Loader, Montero and Lorenzen 2 ). Independently, endothelial dysfunction precedes atherosclerosis( Reference Versari, Daghini and Virdis 3 ), supporting that managing PPH would protect against CVD-related morbidity. In agreement, controlled studies demonstrate that PPH impairs vascular endothelial function (VEF) in an oxidative stress-dependent manner likely by decreasing NO∙ bioavailability( Reference Mah, Noh and Ballard 4 ). VEF is primarily regulated by NO∙ and its diminished bioavailability increases CVD risk( Reference Naseem 5 ). PPH-mediated oxidative stress is centrally implicated in impairing VEF( Reference Mah and Bruno 6 ). Indeed, lipid peroxidation increases immediately following an oral glucose tolerance test (OGTT) in healthy adults( Reference Mah, Noh and Ballard 4 ). Increases in lipid peroxidation are also associated with decreases in brachial artery flow-mediated dilation (FMD), a functional index of NO∙-dependent VEF( Reference Harris, Nishiyama and Wray 7 ) that is prognostic of future CVD-related mortality( Reference Matsuzawa, Kwon and Lennon 8 ). Thus, dietary modifications that target PPH itself and/or limit oxidative stress responses that decrease NO∙ bioavailability would be expected to lower CVD risk by protecting against impairments in VEF.
Endothelial cells synthesise NO∙ in an endothelial NO∙ synthase (eNOS)-dependent manner from L-arginine (ARG)( Reference Naseem 5 ). In addition, arginine:glycine amidinotransferase synthesises homoarginine (hARG), which is a structural homologue of ARG that functions as an alternate substrate for eNOS( Reference Papageorgiou, Androulakis and Papaioannou 9 ). NO∙ biosynthesis is therefore dependent, in part, on substrate availability (i.e. ARG and hARG) for binding to eNOS. However, oxidative stress dysregulates ARG metabolism( Reference Naseem 5 ). Indeed, it increases arginase-mediated catabolism of ARG( Reference Ryoo, Lemmon and Soucy 10 ) while also upregulating protein methyltransferase activity to methylate ARG to asymmetric and symmetric dimethylarginine (ADMA and SDMA)( Reference Boger, Sydow and Borlak 11 ). Both ADMA and SDMA compete with ARG for endothelial cell uptake( Reference Bode-Boger, Scalera and Kielstein 12 , Reference Leiper and Vallance 13 ), whereas ADMA also competitively inhibits eNOS activity( Reference Boger, Sydow and Borlak 11 ). In controlled trials, PPH-induced oxidative stress impairs VEF in association with increases in ADMA normalised to ARG (ADMA/ARG), an index that suggests reduced NO∙ biosynthesis( Reference Mah, Noh and Ballard 4 ). Oxidative stress also decreases arginine:glycine amidinotransferase activity( Reference Ito, Yufu and Mori 14 ), which is expected to lower hARG levels, an effect that is associated with decreased VEF( Reference Valtonen, Laitinen and Lyyra-Laitinen 15 ) and increased CVD risk( Reference Papageorgiou, Androulakis and Papaioannou 9 , Reference Atzler, Schwedhelm and Choe 16 ).
In addition to dysregulating ARG metabolism, other mechanisms exist by which PPH-mediated oxidative stress reduces NO∙ biosynthesis. Oxidation of the eNOS cofactor tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2) results in generation of the superoxide due to eNOS uncoupling( Reference Vasquez-Vivar, Kalyanaraman and Martasek 17 ). In agreement, acute incubation of murine endothelial cells with glucose increases superoxide generation in association with decreased BH4 and increased BH2 ( Reference Crabtree, Smith and Lam 18 ). Importantly, decreased BH4 redox status (i.e. decreased BH4/BH2) is associated with decreased FMD responses( Reference Takeda, Yamashita and Shinohara 19 ), suggesting that BH4/BH2 helps to maintain endothelial function. Hyperglycaemia-mediated increases in angiotensin-II (Ang-II)( Reference Day, de Cássia Cavaglieri and Tabatabaimir 20 ) and endothelin-1 (ET-1)( Reference Grassi, Desideri and Necozione 21 ) may also diminish VEF. In addition to their vasoconstricting activities, Ang-II and ET-1 induce oxidative stress that limits NO∙ biosynthesis and endothelial-dependent vasodilation by decreasing eNOS expression and activity( Reference Loot, Schreiber and Fisslthaler 22 , Reference Ramzy, Rao and Tumiati 23 ). PPH-mediated oxidative stress also induces inflammation( Reference Esposito, Nappo and Marfella 24 ) that exacerbates oxidative stress-mediated depletion of NO∙ ( Reference Siti, Kamisah and Kamsiah 25 ). Thus, PPH is implicated in impairing VEF through several oxidative stress-mediated mechanisms.
Epidemiological evidence supports that PPH is a better predictor of CVD-related mortality compared with fasting glucose( 26 ). Thus, dietary approaches that mitigate PPH-mediated oxidative stress would be expected to lower CVD risk by improving VEF. In support, we have reported in prediabetic men that replacing a portion of an OGTT with isoenergetic amounts of non-carbohydrate containing whole eggs (EGG) or egg whites (WHITE), but not egg yolks (YOLK), alleviates impairments in VEF by limiting PPH( Reference McDonald, Chitchumroonchokchai and Li 27 ). The lack of benefit by the egg yolk-based meal was surprising because, identical to the EGG- and WHITE-based meals, it was 25 % lower in glucose content compared with the OGTT (100 g). This indicates that simply reducing the glucose content alone is not entirely responsible for the observed vasoprotection mediated by EGG or WHITE( Reference McDonald, Chitchumroonchokchai and Li 27 ). Studies in vitro demonstrate that WHITE-derived hydrolysates exhibit free-radical scavenging activity( Reference Davalos, Miguel and Bartolome 28 ) and whole-egg hydrolysates also lower oxidative stress in rodent models( Reference Jahandideh, Majumder and Chakrabarti 29 ). Hydrolysates derived from either EGG or WHITE also induce NO∙-dependent vasorelaxation( Reference Jahandideh, Majumder and Chakrabarti 29 , Reference Matoba, Usui and Fujita 30 ). Thus, in the present study, we hypothesised that vasoprotection following co-ingestion of EGG or WHITE with glucose occurred by limiting PPH-mediated oxidative stress to improve NO∙ bioavailability. We used archived plasma samples collected as part of a previously reported clinical trial( Reference McDonald, Chitchumroonchokchai and Li 27 ) (clinicaltrials.gov; NCT02364570) to test this secondary hypothesis.
Methods
Chemicals and reagents
HPLC-grade solvents and the following chemicals were purchased from Fisher Scientific: acetonitrile, ascorbic acid, butanol, citric acid, diethylenetriaminepentaacetic acid, dithioerythritol, dithiothreitol, ethanol, hexane, hydrochloric acid, methanol, methyl tert-butyl ether, o-phthalaldehyde, perchloric acid, sodium hydroxide, tetrahydrofuran and thiobarbituric acid. The following were from Sigma-Aldrich: β-apo-8-carotenal, biopterin, iodine, monosodium phosphate, potassium iodide, octyl sulphate sodium salt and trichloroacetic acid.
Participants and study design
The study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures were approved by the Institutional Review Board at The Ohio State University (2014H0307), and participants provided written informed consent before enrolling. Complete details of this clinical trial and participant characteristics have been reported previously( Reference McDonald, Chitchumroonchokchai and Li 27 ). In brief, participants were prediabetic based on fasting blood glucose (5·6–6·9 mmol/l)( 31 ), normocholesterolemic (<6·2 mmol/l), weight stable (±2 kg for last 3 months) with a BMI between 25 and 35 kg/m2, and blood pressure ≤140/90 mmHg. They were non-users of dietary supplements or vasoactive medications (>1-month), non-vegetarians, non-smokers, engaged in <7 h/week of aerobic exercise, consumed <3 alcoholic drinks/d and self-reported to be free of CVD. Participants consumed identical egg-free euenergetic diets for 3 d before each intervention, which was reported previously to be verified from written food records( Reference McDonald, Chitchumroonchokchai and Li 27 ).
In this randomised, cross-over clinical trial( Reference McDonald, Chitchumroonchokchai and Li 27 ), men (25–50 years) with prediabetes completed four postprandial trials that were each separated by 7 d. For the present investigation, changes in plasma total NO∙ metabolites (i.e. the sum of nitrate and nitrite), malondialdehyde, antioxidants (vitamins C, E and carotenoids), ARG and endogenous metabolites (hARG, ADMA and SDMA), BH4/BH2, vasoconstrictors (Ang-II and ET-1), inflammatory markers (C-reactive protein (CRP), myeloperoxidase (MPO) and TNF-α) were evaluated during a 3-h postprandial period following the ingestion of isoenergetic test meals. Test meals consisted of 100 g glucose (GLU) alone or 75 g glucose in combination with 1·5 EGG, seven WHITE or two YOLK. Test meals were formulated, as described previously( Reference McDonald, Chitchumroonchokchai and Li 27 ), to be similar to the typical energy content of breakfast consumed by American males (approximately 1670 kJ (400 kcal))( 32 ). In addition, the glucose content in egg-based meals (75 g) was selected on the basis that it reflects the dose used in an OGTT, which has been shown to also induce PPH, oxidative stress and impair VEF( Reference Mah, Noh and Ballard 4 ). Plasma collected before (t=0 min) and at 30, 60, 90, 120, 150 and 180 min after meal ingestion was used for the analyses in the present study. All assessments were performed from 0 to 180 min because PPH transiently impairs VEF during this time period in response to an OGTT( Reference Mah, Noh and Ballard 4 , Reference McDonald, Chitchumroonchokchai and Li 27 ). For measurements of biopterin, plasma was obtained by centrifugation from blood collected in EDTA tubes containing 0·1 % (w/v) dithioerythritol and 0·1 % (w/v) ascorbic acid. For measurements of vitamin C and uric acid, Na heparinised plasma was mixed (1:1) with 10 % (w/v) perchloric acid containing 1 mm diethylenetriaminepentaacetic acid and centrifuged (4°C, 5 min, 15 000 g ). The supernatant was snap-frozen in liquid N2. All samples were stored at −80°C until analysis.
Oxidative stress and antioxidant measurements
Plasma malondialdehyde was measured by HPLC-fluorescence (FL) following thiobarbituric acid derivatisation and butanol extraction as we described( Reference Mah, Noh and Ballard 4 ) on a Shimadzu LC-20XR system equipped with a RF-20AXL FL detector set to 532/553 nm (excitation/emission). Plasma ascorbic acid and uric acid were measured from perchloric acid-treated plasma, and vitamin E (as α- and γ-tocopherol) was measured following saponification and hexane extraction using our separate HPLC-electrochemical detection procedures( Reference Bruno, Dugan and Smyth 33 ). Plasma carotenoids were measured as described( Reference Gleize, Steib and André 34 ) with minor modification. In brief, 200 µl of plasma was mixed with 200 µl of ethanol, 50 µl of β-apo-8-carotenal (60 nm, internal standard) before being extracted three times with 3 ml of methanol–tetrahydrofuran (1:1) and 4 ml of hexane. The extract was evaporated under N2 gas and reconstituted in 150 µl methanol and 150 µl methyl tert-butyl ether before being filtered (0·22 µm) and injected (50 µl) onto the HPLC system. Carotenoids (lutein, zeaxanthin, α- and β-carotene, α- and β-cryptoxanthin, lycopene) were quantified at 450 nm on a Waters 2695 HPLC system equipped with a 2996 photodiode array detector. Separation was performed isocratically (1·0 ml/min) using a YMC C30 column (150×4·6 mm, 3·0 µm) and a mobile phase consisting of methanol–methyl tert-butyl ether–water (96:2:2).
Measurements of nitric oxide homeostasis
Plasma total nitrite and nitrate, the stable end-products of NO∙ ( Reference Ryoo, Lemmon and Soucy 10 ), were assessed by spectrophotometry to estimate NO∙ status in accordance with the manufacturer’s instructions (Cayman Chemical). Plasma ARG and its metabolites (ADMA, SDMA and hARG) were measured by HPLC-FL (340/455 nm, excitation/emission) following o-phthalaldehyde derivatisation on the aforementioned Shimadzu HPLC-FL system as we described( Reference Mah, Noh and Ballard 4 ).
Total biopterin represents the sum of BH4, BH2 and biopterin and is determined using iodine oxidation under acidic and alkaline conditions( Reference Valdes, Arauna and Gonzalez 35 ). Under acidic conditions, both BH4 and BH2 are oxidised to biopterin, whereas only BH2 is oxidised to biopterin under basic conditions. Thus, the difference in biopterin concentrations between acidic and basic oxidation represents BH4 levels( Reference Valdes, Arauna and Gonzalez 35 ). Plasma biopterin was measured as described( Reference Valdes, Arauna and Gonzalez 35 ) with minor modification. In brief, plasma (400 µl) was mixed with 1 m trichloroacetic acid and centrifuged (4°C, 15 min, 20 000 g ). Following centrifugation, two aliquots of supernatant (100 µl each) were used to evaluate biopterin levels following acidic and basic oxidation. For acidic oxidation, 100 µl 0·2 m hydrochloric acid and 10 µl iodine solution (0·9 % (w/v) iodine and 1·8 % (w/v) potassium iodide in 0·1 m hydrochloric acid) were mixed with supernatant. For basic oxidation, 100 µl 0·2 m sodium hydroxide and 10 µl iodine solution (0·9 % (w/v) iodine and 1·8 % (w/v) potassium iodide in 0·1 m sodium hydroxide) were mixed with supernatant. All samples were then incubated in the dark at room temperature for 60 min and subsequently mixed with 10 µl 2 % (w/v) ascorbic acid to reduce excess iodine. Samples were maintained in a refrigerated autosampler (10°C) and injected (30 µl) onto the aforementioned Waters HPLC system equipped with a 474 FL detector (350/450 nm, excitation/emission) as described( Reference Biondi, Ambrosio and De Pascali 36 ), with minor modification. Separation was performed isocratically (0·9 ml/min) on a Phenomenex Kinetex Evo C18 column (250×4·6 mm, 5·0 µm) thermostated to 35°C using a mobile phase composed of 6·5 mm monosodium phosphate, 6 mm citric acid, 1 mm octyl sulphate sodium salt, 2·5 mm diethylenetriaminepentaacetic acid, 1 mm dithiothreitol and 2 % acetonitrile (pH 3·0). Quantification was performed using external standards that were prepared in parallel.
Vasoconstrictors and inflammatory markers
Plasma vasoconstrictors (Ang-II and ET-1) were measured by ELISA (Enzo Life Sciences Inc.) according to the manufacturers’ instructions using a Synergy H1 microplate reader (Biotek Instruments). Plasma CRP and MPO (BioCheck Inc.) and TNF-α (R&D Systems Inc.) were also measured by ELISA.
Statistical methods
The parent study( Reference McDonald, Chitchumroonchokchai and Li 27 ) was powered to detect between-treatment differences in postprandial FMD responses (primary outcome variable; clinicaltrials.gov: NCT02364570), with a power calculation indicating that nine subjects would be needed to reject the null hypothesis with 90 % power (P<0·05). The present study, which utilises archived specimens from these same participants, was powered to detect differences in the lipid peroxidation biomarker malondialdehyde. This biomarker was chosen to test the secondary hypothesis that previously observed improvements in VEF (i.e. FMD) in relation to PPH would be partly attributed to an egg-mediated attenuation in oxidative stress. Although no postprandial studies have examined egg-based meals on malondialdehyde responses, we reported that postprandial malondialdehyde is highly correlated with glycaemic responses (r 0·87; P<0·05)( Reference Mah, Noh and Ballard 4 ). For the present study, based on EGG attenuating glycaemia by approximately 50 % compared with GLU, we predicted an approximately 50 % reduction in malondialdehyde. Thus, a minimum of seventeen subjects would be needed to detect statistically significant between-treatment effects with 80 % power (P<0·05), thereby indicating that our enrollment of twenty participants( Reference McDonald, Chitchumroonchokchai and Li 27 ) was sufficient to appropriately test our secondary hypothesis. Data reported (means with their standard errors) are change (Δ) from baseline (t=0 min) to visualise between-treatment effects for postprandial responses and were analysed using GraphPad Prism (version 7). Time, treatment and time×treatment interaction effects were evaluated using two-way repeated-measures (RM) ANOVA with the Bonferroni’s correction. Postprandial AUC (AUC0–180 min), as the net difference between responses going above (i.e. positive) and below (i.e. negative) baseline, was calculated using the trapezoidal rule. Differences among trials for baseline values as well as AUC0–180 min were assessed using one-way RM ANOVA with the Bonferroni’s post-test. Correlation coefficients (r) between AUC among study variables were calculated using multiple linear regression while controlling for within-subject RM( Reference Bland and Altman 37 ). P<0·05 was considered statistically significant.
Results
Participants and postprandial glycaemic and vascular responses
Complete details of participant characteristics and postprandial glycaemic and FMD responses were reported previously( Reference McDonald, Chitchumroonchokchai and Li 27 ). In brief, men were prediabetic with a fasting glucose of 6·0 (sem 0·1) mmol/l, non-hypertensive (123 (sem 4)/72 (sem 6) mmHg) and normocholesterolemic (5·0 (sem 0·1) mmol/l). They were obese on the basis of mean BMI (31·1 (sem 0·7) kg/m2), but five of the twenty participants had a BMI indicative of overweightness.
Compared with the GLU trial, postprandial increases in glucose and decreases in FMD were attenuated in the EGG and WHITE trials, but not in the YOLK trial( Reference McDonald, Chitchumroonchokchai and Li 27 ). Relative to baseline (0 min), plasma glucose concentrations increased at 30–120 min in GLU and YOLK whereas they only increased at 30–90 min in EGG and WHITE. FMD responses decreased at 30–60 min in GLU and at 30–180 min in YOLK, but only at 30 min in EGG and WHITE. Compared with GLU, the AUC of glucose and FMD were similarly attenuated in the EGG and WHITE trials; the YOLK trial was not different from the GLU trial. Plasma archived from the previously conducted clinical trial( Reference McDonald, Chitchumroonchokchai and Li 27 ) was used to test the current hypothesis that EGG and WHITE protected against PPH-mediated impairments in VEF by attenuating oxidative stress responses that otherwise decrease NO∙ bioavailability. In brief, none of the plasma biomarkers reported below differed at baseline (t=0) among trials (P>0·05; Table 1) with the exception of Ang-II, which was lower in EGG compared with GLU.
Table 1 Baseline values for plasma biomarkers from each intervention arm* (Mean values with their standard errors, n 20)

GLU, 100 g glucose; EGG, whole eggs; WHITE, egg whites; YOLK, egg yolks; MDA, malondialdehyde; NOx, nitric oxide metabolites (nitrate/nitrite); ARG, arginine; hARG, homoarginine; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; Ang-II, angiotensin-II; ET-1, endothelin-1; CRP, C-reactive protein; MPO, myeloperoxidase.
a,b Mean values with unlike superscript letters were significantly different. P>0·05 for all data reported except for Ang-II (P=0·03).
* Total lipid equals the sum of total cholesterol and TAG.
Plasma malondialdehyde
The magnitude of PPH is correlated with increases in oxidative stress( Reference Mah, Noh and Ballard 4 ). Following test meal ingestion, we observed time, treatment and time×treatment effects for malondialdehyde (P<0·001; Fig. 1). Relative to 0 min, malondialdehyde was increased at 30–120 min in the GLU and YOLK trials but only at 30–60 min in the EGG trial and at 30–90 min in the WHITE trial. Compared with GLU, malondialdehyde was increased to a lesser extent at 60–180 min in EGG and at 60–120 min in WHITE, whereas it was not different in YOLK. AUC was also lower (P<0·001) in the EGG and WHITE trials compared with the GLU and YOLK trials; the YOLK trial was not different from the GLU trial. The AUC of malondialdehyde was positively correlated (P<0·05; Table 2) with glucose and negatively correlated with FMD (P=0·01). Data suggest that the co-ingestion of EGG or WHITE with glucose protects against PPH-mediated decreases in vascular function by limiting lipid peroxidation.
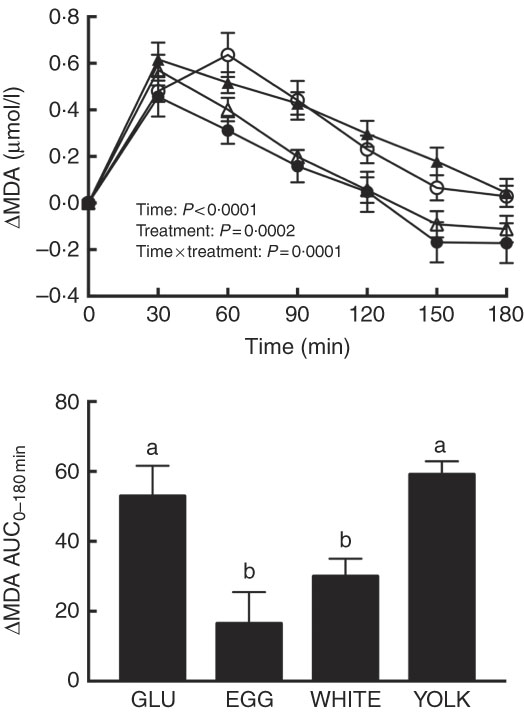
Fig. 1 Postprandial malondialdehyde (MDA) responses and AUC0–180 min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05). GLU (![]() ), 100 g glucose; EGG (
), 100 g glucose; EGG (![]() ), whole eggs; WHITE (
), whole eggs; WHITE (![]() ), egg whites; YOLK (
), egg whites; YOLK (![]() ), egg yolks.
), egg yolks.
Table 2 Pairwise correlations between postprandial AUC0–180 min of study variables in prediabetic men who ingested glucose in the presence or absence of egg-based meals* † ‡
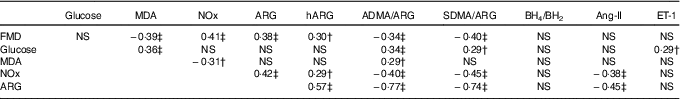
MDA, malondialdehyde; NOx, nitric oxide metabolites (nitrate/nitrite); ARG, arginine; hARG, homoarginine; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; Ang-II, angiotensin-II; ET-1, endothelin-1; FMD, flow-mediated dilation.
* Numerical data represent correlation coefficients (r) between study variables.
† P<0·05.
‡ P≤0·01.
Plasma antioxidants
Plasma antioxidants were assessed to evaluate the extent to which test meals regulated postprandial oxidative stress by altering antioxidant status. Only time effects were observed for postprandial changes in vitamin C and uric acid (P<0·0001, data not shown). Specifically, vitamin C decreased at 30–180 min and uric acid at 150–180 min regardless of trial. However, the AUC of vitamin C and uric acid did not differ among treatments (P>0·05, online Supplementary Table S1). We also assessed lipophilic antioxidants, specifically vitamin E and a panel of carotenoids. Because participants’ lipid responses decreased postprandially regardless of treatment( Reference McDonald, Chitchumroonchokchai and Li 27 ), lipophilic antioxidant concentrations were normalised to total lipid (i.e. the sum of total cholesterol and TAG) as suggested( Reference Gross, Yu and Hannan 38 ). We observed that γ-tocopherol decreased at 120–180 min relative to 0 min (P<0·0001, data not shown) but its AUC as well as that of α-tocopherol did not differ among trials (P>0·05, online Supplementary Table S1). Of the seven carotenoids evaluated (lutein, zeaxanthin, α- and β-carotene, α- and β-cryptoxanthin, lycopene), we only detected a time effect for β-cryptoxanthin and a treatment effect for lutein. β-Cryptoxanthin was increased at 180 min relative to 0 min (P<0·01, data not shown). Lutein was significantly lower in YOLK at 30 min and at 120–150 min compared with EGG and at 30–180 min compared with WHITE (P=0·01, data not shown). However, the AUC of lutein showed no treatment differences despite a significant main effect (P=0·02, online Supplementary Table S1). Thus, it is likely that EGG- and WHITE-based test meals attenuated lipid peroxidation independent of improved antioxidant status.
Plasma nitric oxide metabolites
FMD largely reflects NO∙-dependent vasodilation( Reference Harris, Nishiyama and Wray 7 ), suggesting that improvements in vascular function by EGG and WHITE were attributed to greater NO∙ bioavailability. Plasma NO∙ metabolites (i.e. the sum of nitrite and nitrate), an index of NO∙ bioavailability, showed main and interactive effects (P≤0·01; Fig. 2). Relative to 0 min, NO∙ metabolites were lower at 60–180 min in the GLU trial but were not different from 0 min in response to any egg-based meals. Compared with GLU, NO∙ metabolites decreased to a lesser extent at 60–150 min in the EGG trial, at 60–90 and 150 min in the WHITE trial, but only at 60 min in the YOLK trial. AUC was similarly greater in the EGG and WHITE trials compared with the GLU trial (P<0·01), whereas the YOLK trial was not different from the GLU trial (P>0·05). NO∙ metabolites positively correlated with FMD (P<0·01, Table 2), suggesting that improved VEF was due to greater NO∙ bioavailability. An inverse relationship between NO∙ metabolites and malondialdehyde (P<0·05) was also observed, consistent with oxidative stress diminishing NO∙ bioavailability. Data suggest that EGG and WHITE protect against impairments in VEF, at least in part, by preserving NO∙ bioavailability that is otherwise decreased by PPH-mediated oxidative stress.
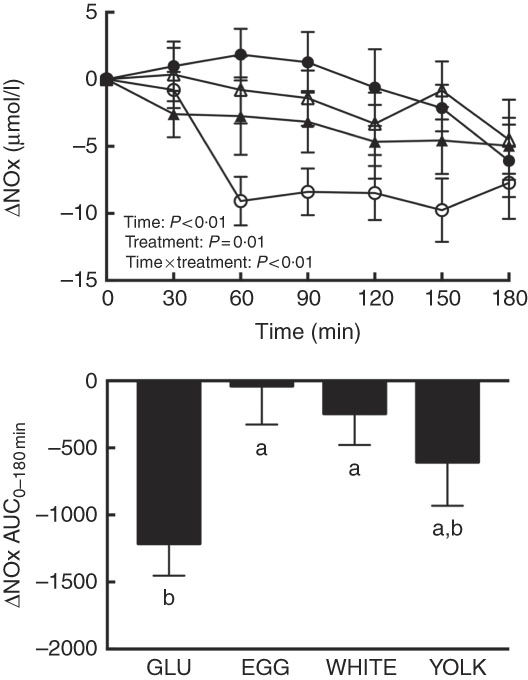
Fig. 2 Postprandial nitric oxide metabolites (nitrate/nitrite) (NOx) responses and AUC0–180 min following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05). GLU (![]() ), 100 g glucose; EGG (
), 100 g glucose; EGG (![]() ), whole eggs; WHITE (
), whole eggs; WHITE (![]() ), egg whites; YOLK (
), egg whites; YOLK (![]() ), egg yolks.
), egg yolks.
l-Arginine and homoarginine availability
Postprandial changes in ARG and hARG were assessed to test the hypothesis that improved NO∙ status during EGG and WHITE (Fig. 2) was attributed to greater substrate availability. We observed main and interactive effects for both ARG and hARG (P<0·01; Fig. 3(A) and (B)). Relative to 0 min, ARG was decreased at 30–180 min in the GLU trial, at 60–180 min in the EGG trial and at 90–180 min in the YOLK trial (Fig. 3(A)). However, ARG was increased at 150 min in the WHITE trial. Decreases in ARG otherwise occurring in GLU were attenuated at 60–180 min in EGG and at 30–180 min in YOLK. In addition, ARG was increased at 90–180 min in WHITE compared with GLU. The AUC of ARG was similarly greater in EGG and YOLK, but even greater in WHITE (P<0·0001). ARGAUC was also positively correlated with NO∙ metabolites and FMD (P<0·01; Table 2).
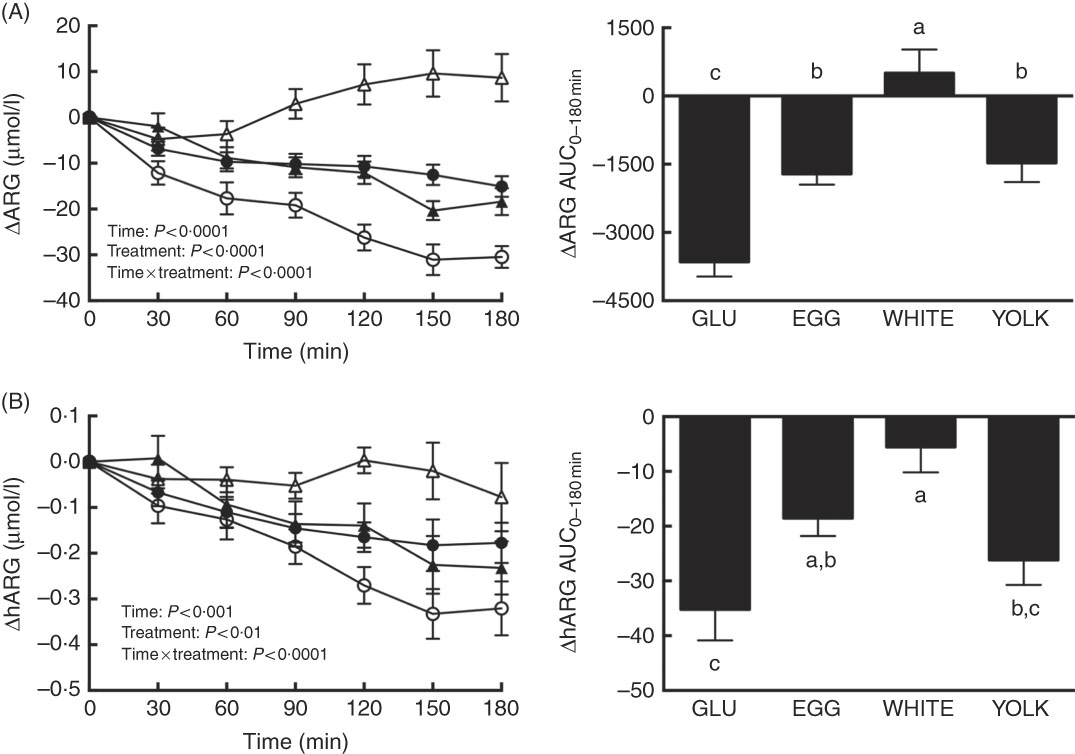
Fig. 3 Postprandial responses and AUC0–180 min of (A) l-arginine (ARG) and (B) homoarginine (hARG) following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05). GLU (![]() ), 100 g glucose; EGG (
), 100 g glucose; EGG (![]() ), whole eggs; WHITE (
), whole eggs; WHITE (![]() ), egg whites; YOLK (
), egg whites; YOLK (![]() ), egg yolks.
), egg yolks.
Relative to baseline levels, hARG was decreased at 90–180 min in the GLU, EGG and YOLK trials but were unaffected in the WHITE trial (Fig. 3(B)). Compared with GLU, decreases in hARG were attenuated at 120 min in YOLK, at 150–180 min in EGG and at 90–180 min in WHITE. The AUC of hARG was greater in EGG and greatest in WHITE compared with GLU (P=0·0001); YOLK did not differ from GLU (P>0·05). The AUC of hARG was positively correlated (P<0·05, Table 2) with the AUC of ARG, NO∙ metabolites and FMD. Thus, co-ingestion of EGG or WHITE with glucose may in part protect against declines in NO∙ bioavailability by improving substrate availability for eNOS.
Competitive inhibition of endothelial nitric oxide synthase activity and l-arginine uptake
We assessed changes in the ARG metabolites ADMA and SDMA to consider the possibility that greater NO∙ metabolites during the EGG and WHITE trials were attributed to limiting competitive inhibition of eNOS by ADMA( Reference Boger, Sydow and Borlak 11 ) and arginine uptake by ADMA and SDMA( Reference Bode-Boger, Scalera and Kielstein 12 , Reference Leiper and Vallance 13 ). For ADMA/ARG as well as SDMA/ARG, we observed effects due to time, treatment and their interaction (P<0·0001; Fig. 4(A) and (B)). ADMA/ARG (Fig. 4(A)) was increased at 30–180 min in the GLU and YOLK trials and at 30–60 min in the EGG trial relative to 0 min. ADMA/ARG was also increased at 30 min in the WHITE trial and decreased relative to baseline at 120–180 min. ADMA/ARG increased to a lesser extent at 60–180 min in the WHITE trial and at 90–180 min in the EGG and YOLK trials compared with GLU. Likewise, the AUC of ADMA/ARG was lower in EGG and YOLK and lowest in WHITE compared with GLU (P<0·0001). ADMA/ARGAUC was inversely correlated (P≤0·01; Table 2) with AUC of NO∙ metabolites, ARG and FMD, but positively correlated (P<0·05) with AUC of glucose and malondialdehyde. This suggests that the observed vasoprotection in the EGG and WHITE trials was attributed to, at least in part, greater NO∙ bioavailability that was otherwise reduced by competitive inhibition of eNOS.
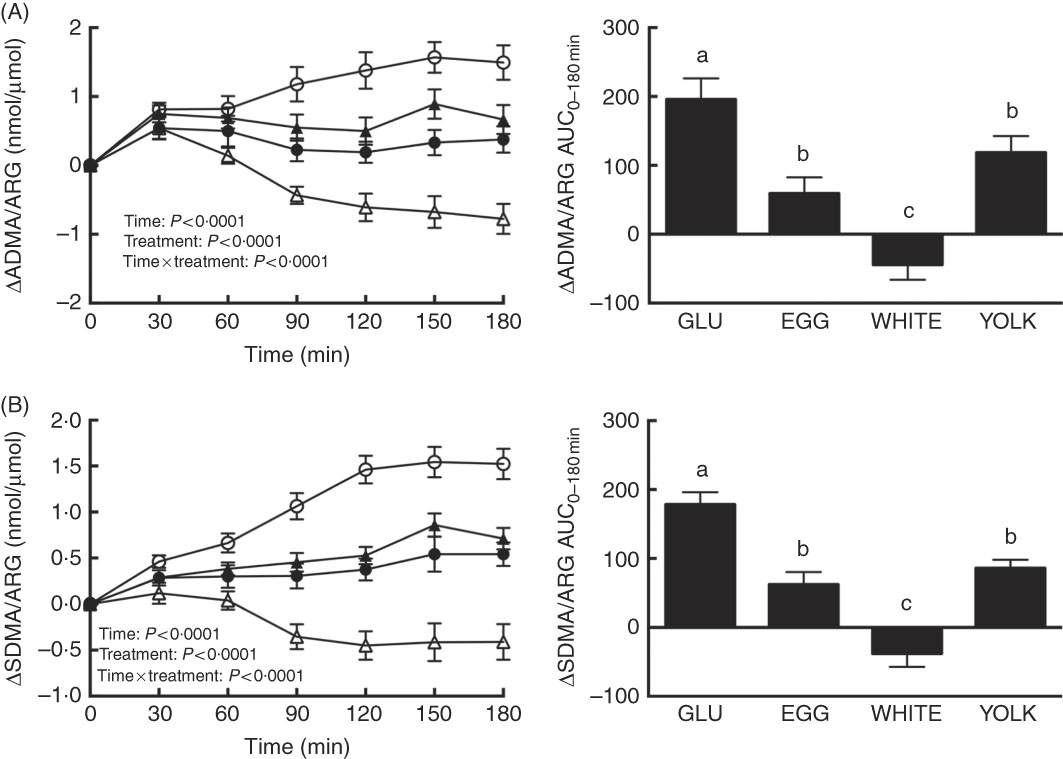
Fig. 4 Postprandial responses and AUC0–180 min of (A) asymmetric dimethylarginine (ADMA)/l-arginine (ARG) and (B) symmetric dimethylarginine (SDMA)/ARG following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P<0·05). GLU (![]() ), 100 g glucose; EGG (
), 100 g glucose; EGG (![]() ), whole eggs; WHITE (
), whole eggs; WHITE (![]() ), egg whites; YOLK (
), egg whites; YOLK (![]() ), egg yolks.
), egg yolks.
Relative to 0 min, the SDMA:ARG ratio (Fig. 4(B)) increased at 30–180 min in the GLU trial, at 120–180 min in the EGG trial and at 60–180 min in the YOLK trial, whereas it decreased at 120–180 min in the WHITE trial. Relative to the GLU trial, SDMA/ARG was attenuated at 30–180 min in the WHITE trial, at 60–180 min in the EGG trial and at 90–180 min in the YOLK trial. The SDMA/ARGAUC was also similarly lower in the EGG and YOLK trials but lowest in the WHITE trial (P<0·0001) compared with the GLU trial. SDMA/ARGAUC was inversely correlated (P<0·01; Table 2) with AUC of NO∙ metabolites, ARG and FMD, but positively correlated (P<0·05) with glucoseAUC. Thus, improvements in NO∙ bioavailability and vascular function by EGG and WHITE may occur, in part, by improving ARG uptake into endothelial cells.
Tetrahydrobiopterin redox status
Postprandial BH4 redox status was assessed to determine if improved NO∙ status in the EGG and WHITE trials was attributed to attenuating eNOS uncoupling( Reference Crabtree, Smith and Lam 18 ). However, changes in plasma concentrations of BH4, BH2 and BH4/BH2 and their respective AUC did not differ among trials (P>0·05, online Supplementary Fig. S1).
Plasma vasoconstrictors and inflammatory markers
Ang-II and ET-1 and inflammatory markers were assessed postprandially to define their potential contribution to reduce NO∙ bioavailability. We observed time, treatment and interaction effects for Ang-II (P<0·0001; Fig. 5(A)). Relative to 0 min, Ang-II increased at 30–180 min in the GLU trial but only at 60–120 min in all egg-based trials. GLU-induced increases in Ang-II were similarly attenuated at 30–180 min in egg-based trials. The AUC of Ang-II was also similarly lower in egg-based treatments compared with the GLU trial (P<0·0001). Ang-IIAUC negatively correlated (P<0·01; Table 2) with AUC of NO∙ metabolites and ARG.
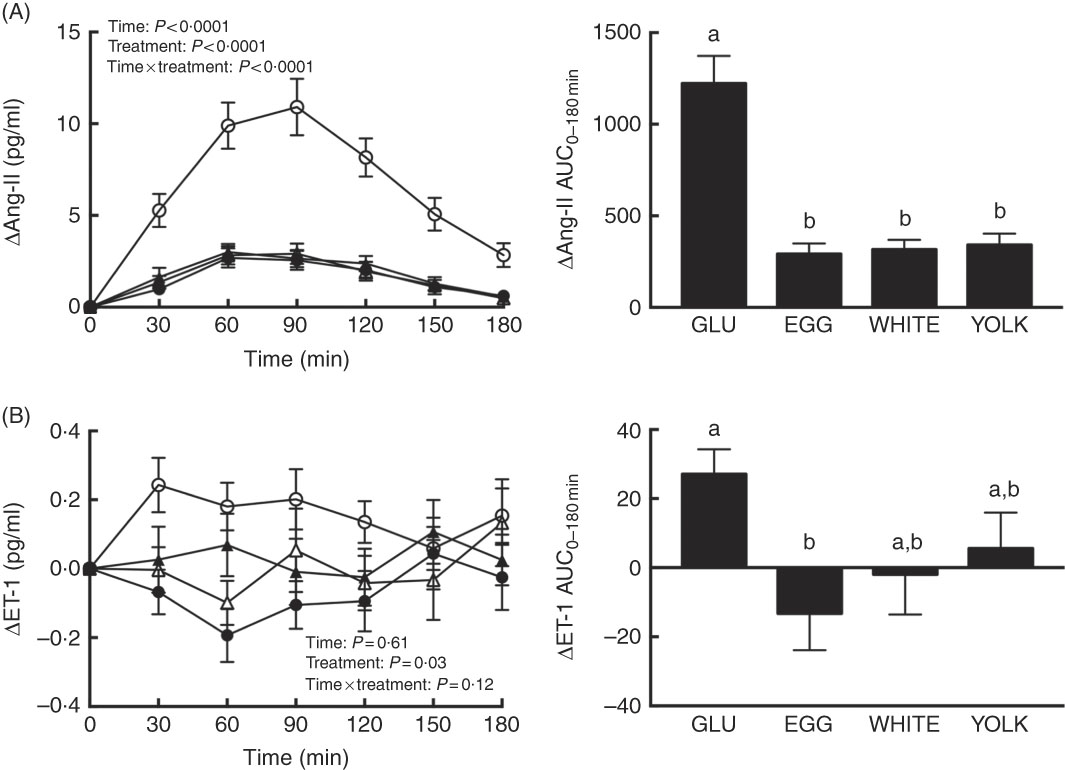
Fig. 5 Postprandial responses and AUC0–180 min of (A) angiotensin-II (Ang-II) and (B) endothelin-1 (ET-1) following ingestion of glucose in the absence or presence of egg-based meals by prediabetic men. Postprandial responses were analysed using two-way repeated-measures (RM) ANOVA with Bonferroni’s post hoc test. AUC0–180 min was calculated using the trapezoidal rule and analysed using one-way RM ANOVA with Bonferroni’s post hoc test. Values are means (n 20), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05). GLU (![]() ), 100 g glucose; EGG (
), 100 g glucose; EGG (![]() ), whole eggs; WHITE (
), whole eggs; WHITE (![]() ), egg whites; YOLK (
), egg whites; YOLK (![]() ), egg yolks.
), egg yolks.
Plasma ET-1 showed an effect due to treatment only (P=0·03; Fig. 5(B)). Although plasma ET-1 increased to the greatest extent in the GLU trial, its concentrations were attenuated at 30–60 min in the WHITE trial and at 30–90 and 180 min in the EGG trial; the YOLK trial was not different from the GLU trial. The AUC of ET-1 was only lower in EGG compared with GLU (P<0·05). In addition, ET-1AUC and glucoseAUC were positively correlated (P<0·05; Table 2). Plasma CRP, MPO and TNF-α and their respective AUC did not differ among trials (P>0·05, online Supplementary Fig. S2). This suggests that greater NO∙ bioavailability by EGG and WHITE may be attributed to attenuating ET-1 independent of Ang-II and inflammation.
Discussion
Our prior study in this cohort of men with prediabetes demonstrated that replacing 25 % of an OGTT (100 g) with an isoenergetic amount of EGG or WHITE protected against PPH-mediated impairments in FMD( Reference McDonald, Chitchumroonchokchai and Li 27 ). However, reducing the glucose content of the OGTT and replacing it with YOLK did not confer vasoprotection. This indicates that reducing the oral glucose load alone does not fully explain EGG- and WHITE-mediated vasoprotection( Reference McDonald, Chitchumroonchokchai and Li 27 ). Indeed, the present study provides novel evidence that the previously observed vasoprotection in EGG and WHITE is mediated, in part, by attenuating dysregulated ARG metabolism and oxidative stress that otherwise decrease NO∙ bioavailability. Data show greater NO∙ metabolites in association with greater ARG and hARG and lower ADMA/ARG and SDMA/ARG. Although all egg-based meals protected against dysregulated ARG metabolism, which would be expected to improve NO∙ biosynthesis, NO∙ metabolites and FMD responses were only improved in the EGG and WHITE trials. The lack of vasoprotection observed in the YOLK trial is likely attributed to its inability to limit lipid peroxidation. Indeed, lower malondialdehyde, in association with greater NO∙ metabolites, occurred in the EGG and WHITE trials, but not in the YOLK trial. Moreover, limiting increases in malondialdehyde was correlated with improvements in NO∙ metabolites and FMD, whereas greater NO∙ metabolites was correlated with improved FMD responses. This suggests that vasoprotection in the EGG and WHITE trials was mediated by alleviating PPH-induced oxidative stress that otherwise impairs NO∙-dependent vascular function. This is likely attributed to attenuating both dysregulated ARG metabolism and oxidative stress that otherwise reduce NO∙ biosynthesis and bioavailability to impair VEF (Fig. 6).
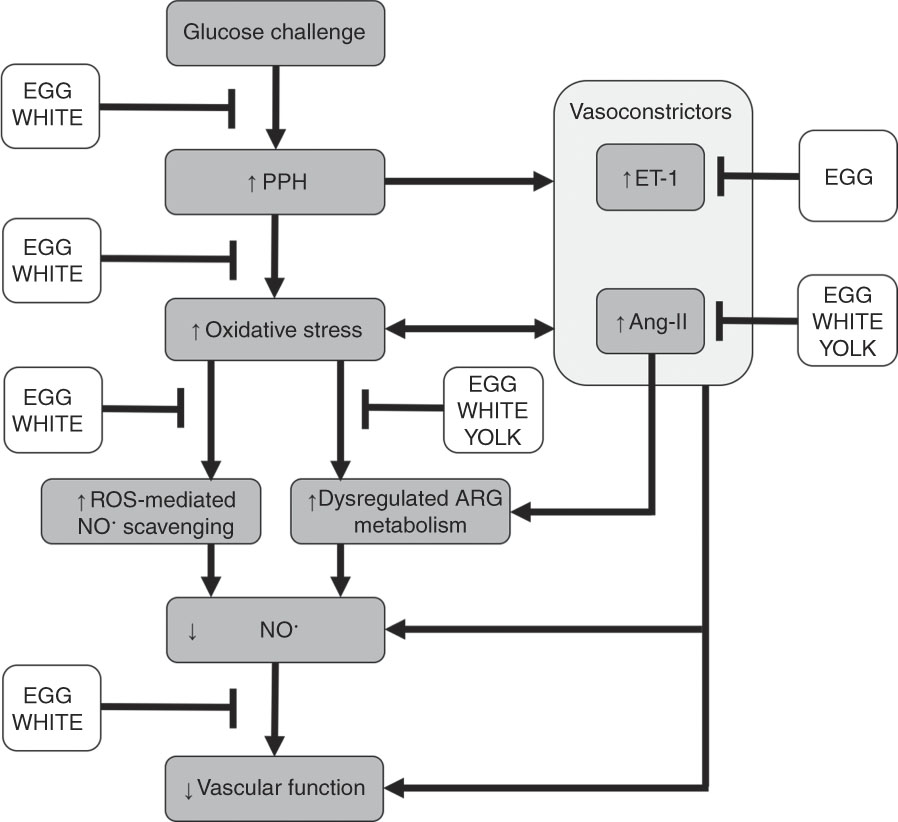
Fig. 6 Summary of the vasoprotective activities of egg-based meals on postprandial hyperglycaemia (PPH)-mediated impairments in vascular function. Ingestion of an oral glucose challenge induces PPH, which increases the vasoconstrictors angiotensin-II (Ang-II) and endothelin-1 (ET-1) while also inducing oxidative stress. Ang-II and ET-1 also contribute to oxidative stress and are upregulated by oxidative stress. Consequently, oxidative stress decreases nitric oxide bioavailability via the direct scavenging of nitric oxide by reactive oxygen species (ROS) and impairing nitric oxide biosynthesis by dysregulating arginine (ARG) metabolism. Collectively, decreases in nitric oxide impair vascular function. Co-ingestion of whole eggs (EGG) or egg whites (WHITE) with glucose attenuates PPH-mediated oxidative stress. This alleviates dysregulated ARG metabolism and scavenging of nitric oxide that otherwise reduces nitric oxide biosynthesis and bioavailability to impair vascular function. Co-ingesting egg yolks (YOLK) with glucose only attenuates dysregulated ARG metabolism but does not attenuate PPH-mediated oxidative stress, likely allowing for ROS-mediated nitric oxide scavenging and an inability to maintain vascular function. Egg-based meals differentially affect Ang-II and ET-1, which provoke dysregulated ARG metabolism, decrease nitric oxide and impair vascular function. Overall, vasoprotection mediated by whole eggs and egg whites is likely attributed to their benefits on dysregulated ARG metabolism and ROS-mediated scavenging, which improve nitric oxide bioavailability to the vasculature.
Studies in vitro demonstrate that hydrolysates derived from WHITE exhibit free-radical scavenging activity( Reference Liu, Oey and Bremer 39 ) and those from EGG lower malondialdehyde concentrations in rodents( Reference Jahandideh, Majumder and Chakrabarti 29 ). This would be expected to attenuate oxidative stress-mediated declines in NO∙ bioavailability. In addition, peptides resulting from simulated digestion of EGG and WHITE induce ex vivo endothelium-dependent vasodilation in mesenteric arteries isolated from spontaneously hypertensive rats( Reference Jahandideh, Majumder and Chakrabarti 29 , Reference Matoba, Usui and Fujita 30 ). In hyperlipidaemic adults, 6 weeks daily WHITE consumption increased fasting FMD responses( Reference Njike, Faridi and Dutta 40 ), which suggests improved NO∙ bioavailability, but this was not reported. Previously reported improvements in FMD( Reference McDonald, Chitchumroonchokchai and Li 27 ) provided rationale for assessing NO∙ bioavailability in the present study. Indeed, increases in malondialdehyde that were observed in the GLU trial were attenuated in the EGG and WHITE trials in association with decreases in NO∙ metabolites and FMD. This suggests that limiting PPH-induced oxidative stress improves NO∙ bioavailability and NO∙-dependent vascular function. In support, NO∙ metabolites positively correlated with FMD, indicating greater levels of NO∙ metabolites were accompanied with improved vascular function. Shear stress, or the force of blood flow tangential to the endothelial surface, depending on the flow pattern, can promote NO∙ or induce oxidative stress( Reference Chatzizisis, Coskun and Jonas 41 ). Thus, differences in shear stress were considered to explain EGG- and WHITE-mediated vasoprotection. However, as reported previously, shear stress did not differ among treatments( Reference McDonald, Chitchumroonchokchai and Li 27 ).
Oxidative stress is implicated in decreasing ARG availability, in part, by upregulating arginase-dependent catabolism( Reference Ryoo, Lemmon and Soucy 10 ). Lower ARG availability would be expected to decrease NO∙ biosynthesis( Reference Naseem 5 ) and impair VEF( Reference Preli, Klein and Herrington 42 ). In agreement, we show that increases in malondialdehyde during the GLU trial were accompanied by decreases in ARG. NO∙ metabolites and FMD were also correlated with ARG, suggesting that vasoprotection in the EGG and WHITE trials occurred, in part, by improving ARG availability for NO∙ biosynthesis. This is consistent with ARG supplementation improving VEF( Reference Preli, Klein and Herrington 42 ). Despite malondialdehyde not differing between the GLU and YOLK trials, and being higher than that in the EGG trial, postprandial ARG was not different between EGG and YOLK trials. This suggests that greater lipid peroxidation in YOLK compared with EGG does not fully explain ARG status. We therefore considered that circulating ARG was influenced by the dietary ARG content of test meals( Reference Mariotti, Huneau and Szezepanski 43 ). In support, ARG increased to the greatest extent in the WHITE trial, the treatment that also provided the greatest dietary ARG (1·497 g)( 44 ). However, postprandial ARG decreased and was not different between the EGG (0·615 g) and YOLK (0·374 g) trials despite an approximately 40 % difference in dietary ARG. While differences in dietary ARG may help to explain increases observed in the WHITE trial, it fails to address the lack of difference between EGG and YOLK trials. Regardless, improvements in ARG status in the YOLK trial would be expected to increase NO∙ biosynthesis, but this was not observed. The lack of benefit of YOLK on NO∙ metabolites is likely attributed to its inability to limit lipid peroxidation otherwise induced by PPH. This suggests that oxidative stress-mediated scavenging of NO∙ precluded its bioavailability to the vascular endothelium, which is consistent with the lack of improvement in vascular function in the YOLK trial( Reference McDonald, Chitchumroonchokchai and Li 27 ). In contrast, FMD responses were improved in the EGG and WHITE trials( Reference McDonald, Chitchumroonchokchai and Li 27 ), which was accompanied by greater NO∙ metabolites and lower lipid peroxidation. Based on these findings, future study is warranted to define the independent and additive effects of egg components to regulate ARG status and NO∙ bioavailability in relation to VEF.
Oxidative stress also decreases arginine:glycine amidinotransferase activity( Reference Ito, Yufu and Mori 14 ), which would be expected to lower hARG to limit NO∙ biosynthesis( Reference Papageorgiou, Androulakis and Papaioannou 9 ) and impair vascular function( Reference Valtonen, Laitinen and Lyyra-Laitinen 15 ). In support, increases in malondialdehyde during the GLU trial were accompanied by decreases in hARG that were attenuated in the EGG and WHITE trials, but not in the YOLK trial. Furthermore, hARG was positively correlated with NO∙ metabolites and FMD. These findings are in agreement with evidence that higher hARG levels are associated with greater FMD responses( Reference Valtonen, Laitinen and Lyyra-Laitinen 15 ) and lower CVD risk( Reference Papageorgiou, Androulakis and Papaioannou 9 , Reference Atzler, Schwedhelm and Choe 16 ). However, regulation of VEF by hARG is an area of active investigation due to disparate observations( Reference Papageorgiou, Androulakis and Papaioannou 9 , Reference Atzler, Schonhoff and Cordts 45 ). Despite its ability to serve as an eNOS substrate and inhibit arginase activity, hARG has less binding affinity for eNOS( Reference Moali, Boucher and Sari 46 ) and physiological levels (approximately 2 µm) are approximately 10–100-fold less than ARG( Reference Michel 47 ). Thus, attenuation of oxidative stress in the EGG and WHITE trials may have improved hARG status, but this may not explain the vasoprotective benefits of EGG and WHITE.
Oxidative stress also increases the accumulation of ADMA and SDMA by upregulating protein methyltransferase( Reference Boger, Sydow and Borlak 11 ) while preventing dimethylarginine dimethylaminohydrolase-mediated catabolism of ADMA( Reference Mah and Bruno 6 ). Likewise, alanine-glyoxylate aminotransferase-2 also catabolises ADMA and SDMA( Reference Tain and Hsu 48 ) but the role of oxidative stress on alanine-glyoxylate aminotransferase-2 activity has not been reported. Oxidative stress decreases the activity of cationic amino acid transporter to limit cellular clearance of ADMA and SDMA( Reference Tain and Hsu 48 , Reference Luo, Teerlink and Griendling 49 ). Collectively, this decreases NO∙ biosynthesis( Reference Naseem 5 ), consistent with ADMA and SDMA competitively inhibiting cellular uptake of ARG( Reference Bode-Boger, Scalera and Kielstein 12 , Reference Leiper and Vallance 13 ) and ADMA competitively inhibiting eNOS( Reference Boger, Sydow and Borlak 11 ). In agreement, we show that increases in malondialdehyde during the GLU trial were accompanied by increases in ADMA/ARG and SDMA/ARG that were similarly attenuated by EGG and WHITE. In addition, ADMA/ARG and SDMA/ARG negatively correlated with NO∙ metabolites and FMD, suggesting that PPH-mediated oxidative stress limited NO∙ biosynthesis to impair vascular function by inhibiting cellular ARG uptake and eNOS activity. Our findings are consistent with those demonstrating that PPH impaired VEF, in part, by increasing ADMA/ARG following an OGTT( Reference Mah, Noh and Ballard 4 ). They are also consistent with evidence in vitro showing that SDMA dose-dependently increases oxidative stress and reduces NO∙ metabolites otherwise abrogated by ARG( Reference Bode-Boger, Scalera and Kielstein 12 ). This would be expected to impair VEF and is supported by evidence associating greater SDMA with increased CVD risk in diabetic subjects( Reference Zobel, von Scholten and Reinhard 50 ). Interestingly, compared with the GLU trial, increases in ADMA/ARG and SDMA/ARG were attenuated in the YOLK trial despite no improvements in malondialdehyde. However, this did not improve NO∙ metabolites or FMD responses and suggests that improving NO∙ biosynthesis alone does not fully confer vasoprotection. Rather, decreasing oxidative stress-mediated scavenging of NO∙ is also needed to facilitate NO∙ bioavailability and maintain VEF (Fig. 6). Improvements in ADMA/ARG and SDMA/ARG suggest bioactive egg components may inhibit protein methyltransferase or increase activities of dimethylarginine dimethylaminohydrolase, alanine-glyoxylate aminotransferase-2 and/or cationic amino acid transporter but requires future investigation.
Apart from dysregulating ARG metabolism, PPH-mediated oxidative stress also impairs vascular function by oxidising the eNOS cofactor BH4 ( Reference Crabtree, Smith and Lam 18 , Reference Ihlemann, Rask-Madsen and Perner 51 ) and by increasing Ang-II( Reference Day, de Cássia Cavaglieri and Tabatabaimir 20 , Reference Keidar, Heinrich and Kaplan 52 ) and ET-1( Reference Grassi, Desideri and Necozione 21 , Reference Ruef, Moser and Kubler 53 ) to decrease eNOS expression and activity( Reference Loot, Schreiber and Fisslthaler 22 , Reference Ramzy, Rao and Tumiati 23 ). Thus, attenuation of oxidative stress in the EGG and WHITE trials, but not the YOLK trials, may have conferred vasoprotection through these pathways. However, BH4/BH2 was unaffected by treatment, suggesting increased oxidative stress was independent of eNOS uncoupling( Reference Vasquez-Vivar, Kalyanaraman and Martasek 17 ). This may be due to participants having adequate vitamin C concentrations (approximately 40 μm)( Reference Schleicher, Carroll and Ford 54 ) to recycle BH4 ( Reference Kuzkaya, Weissmann and Harrison 55 ). All egg-based meals protected against increases in Ang-II, which may be due to egg-derived peptides inhibiting angiotensin-converting enzyme( Reference Liu, Oey and Bremer 39 ). In bovine aortic endothelial cells, Ang-II upregulates arginase to decrease NO∙ generation( Reference Shatanawi, Lemtalsi and Yao 56 ). In addition, incubation of rat vascular smooth muscle cells with Ang-II increased protein methyltransferase and decreased dimethylarginine dimethylaminohydrolase and cationic amino acid transporter activity leading to increased ADMA levels. Thus, protection against increases in Ang-II by egg-based meals may have limited Ang-II-mediated decreases in ARG and increases in ADMA and SDMA (Fig. 6), but this requires future study. Lastly, despite evidence showing that PPH increases pro-inflammatory mediators( Reference Esposito, Nappo and Marfella 24 ) and egg ingestion (2–3 eggs/d for 4 weeks) improves antioxidant status( Reference DiMarco, Norris and Millar 57 ), these responses were unaffected in the present study. Thus, vasoprotection in the EGG and WHITE trials, at least postprandially, occurred independent of lowering pro-inflammatory mediators or improving antioxidant status.
Oxidative stress mediated by acute hyperglycaemia plays a central role in reducing NO∙ bioavailability and impairing VEF( Reference Cai and Harrison 58 ). Co-ingestion of EGG or WHITE, but not YOLK, with an OGTT attenuates increases in glycaemia and decreases in FMD responses( Reference McDonald, Chitchumroonchokchai and Li 27 ). The observed vasoprotection was mediated, in part, at the level of the gut by increasing cholecystokinin( Reference McDonald, Chitchumroonchokchai and Li 27 ), which functions to delay gastric emptying( Reference Liddle, Morita and Conrad 59 ). This supports a pre-absorptive mechanism to attenuate glycaemia and downstream oxidative stress. Together with the present study, the available evidence suggests a link between gut-level responses and vascular health. Hyperglycaemia induces oxidative stress through multiple mechanisms including mitochondrial superoxide production, proinflammatory mediators, advanced glycation end products, eNOS uncoupling and increased enzymatic (i.e. NADPH oxidase and protein kinase C) activity( Reference Mah and Bruno 6 , Reference Brownlee 60 ). However, evidence in vitro indicates that hyperglycaemia-mediated activation of these pathways is ameliorated by blocking glucose-induced mitochondrial superoxide production( Reference Nishikawa, Edelstein and Du 61 ). Furthermore, mitochondrial-derived reactive oxygen species activates NADPH oxidase, which in turn, stimulates mitochondrial reactive oxygen species generation, thereby creating a detrimental cycle of oxidative stress( Reference Daiber, Di Lisa and Oelze 62 ). Thus, vasoprotection in the EGG and WHITE trials, but not the YOLK trial, may be potentially attributed to limiting glucose-induced mitochondrial reactive oxygen species production and/or NADPH oxidase activation, which requires further study. In addition, increased malondialdehyde in the YOLK trial may be, in part, due to oxidation of egg yolk lipids during digestion( Reference Kobayashi, Sasahara and Yoda 63 ), which could contribute to NO∙ scavenging and limit its bioavailability for improving VEF. However, increases in malondialdehyde were attenuated in the EGG trial, which may be attributed to WHITE limiting egg yolk lipid oxidation( Reference Kobayashi, Sasahara and Yoda 63 ). Ultimately, limiting reactive oxygen species (e.g. superoxide), which reacts with NO∙, would be expected to increase NO∙ bioavailability to improve VEF.
Our findings show that PPH and lipid peroxidation were attenuated, and vascular function improved in the EGG and WHITE trials only. In addition, there were no statistically different outcomes between the EGG and WHITE trials, suggesting that the WHITE fraction of EGG is functionally responsible for vasoprotection observed previously( Reference McDonald, Chitchumroonchokchai and Li 27 ). In support, peptides resulting from enzymatic digestion of WHITE proteins (e.g. ovalbumin, ovotransferrin, ovomucin and lysozyme) exhibit glycaemia-lowering, antioxidant, and NO∙-producing activities( Reference Liu, Oey and Bremer 39 ). Thus, in addition to cholecystokinin-mediated delays in gastric emptying, bioactive peptides resulting from digestion may mediate a post-absorptive vasoprotective mechanism. However, these peptides and their circulating levels have not been studied in humans. In addition, future postprandial studies should examine other protein-rich foods (e.g. milk) to determine if vasoprotection is unique to eggs. The present study was designed to deliver isoenergetic meals reflective of typical breakfast intakes by American men( Reference McDonald, Chitchumroonchokchai and Li 27 ), and future studies should consider potential dose-dependent effects of eggs and how modifying meal composition with protein and fat regulates postprandial responses. Our studies were specifically limited to a 180-min postprandial period consistent with glucose ingestion transiently impairing vascular function( Reference Mah, Noh and Ballard 4 , Reference McDonald, Chitchumroonchokchai and Li 27 ). However, some of the biomarkers reported herein (i.e. ARG and metabolites) did not return to baseline levels by 180 min. Thus, future studies of longer duration are needed to evaluate the effects of subsequent meals on postprandial health. Lastly, chronic egg consumption attenuates PPH that is induced by an OGTT( Reference Pearce, Clifton and Noakes 64 ). It also decreases fasting malondialdehyde( Reference Jahandideh, Majumder and Chakrabarti 29 ) and increases FMD responses( Reference Njike, Faridi and Dutta 40 ). Thus, chronic studies are needed to examine egg consumption on postprandial glycaemia and oxidative stress in relation to VEF. However, a benefit of postprandial studies is that they permit the direct evaluation of food and its components that is often confounded by the displacement of other foods during chronic studies.
In conclusion, the present study demonstrates that co-ingesting EGG or WHITE, but not YOLK, with an oral glucose challenge protects against postprandial impairments in vascular function by limiting PPH-induced oxidative stress that otherwise scavenges NO∙ to decrease its bioavailability (Fig. 6). Thus, replacing carbohydrates in a meal for EGG or WHITE may be a beneficial dietary strategy to attenuate PPH-mediated oxidative stress that otherwise decreases NO∙ bioavailability to impair vascular function and increase CVD risk. Consistent with PPH being a better predictor of long-term CVD risk compared with fasting glucose( 26 ), a need exists to validate dietary strategies that limit meal-induced injuries to the vascular endothelium that are attributed to PPH and its downstream oxidative stress responses. In the absence of effective approaches, the accumulation of postprandial insults is expected to increase CVD risk, which remains the leading cause of mortality in the USA( Reference Mozaffarian, Benjamin and Go 1 ).
Acknowledgements
The authors thank Meredith Moller and John Bouranis for their assistance with biochemical analyses.
The present study was funded by the American Egg Board/Egg Nutrition Center. American Egg Board/Egg Nutrition Center had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: R. S. B., J. S. V. and E. M. were responsible for the study design; J. D. M., C. C., E. J. R., J. L. and F. A. V. were responsible for collecting and analysing data; J. D. M. and R. S. B. wrote the initial draft of the manuscript and all authors contributed to the editing and review of this manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002192













