Microbial infections including pneumonia, kidney infection, cellulitis and meningitis can trigger sepsis( Reference Dellinger, Levy and Rhodes 1 ), which is a life-threatening organ dysfunction resulting from a dysregulated host response to infection( Reference Shankar-Hari, Phillips and Levy 2 ). Sepsis is responsible for approximately 200 000 deaths in the USA annually( Reference Angus, Linde-Zwirble and Lidicker 3 ). In 2014, there were over 750 000 hospitalisations for sepsis in the USA( Reference McDermot, Elixhauser and Sun 4 ).
Research reveals that diet may play an integral role in influencing sepsis risk. Previous studies have shown that high-fat diets fed to mice are associated with increased mortality, organ injury, susceptibility to sepsis and disturbance in innate immune functions( Reference Kaplan, Nowell and Lahni 5 – Reference Strandberg, Verdrengh and Enge 7 ). Although this study was done in mice and may not be applicable for humans, a disturbance in innate immune functions can affect the body’s ability to combat microbial infections( Reference Ponton, Wilson and Cotter 8 ), which as mentioned above can trigger sepsis. A study, in humans, demonstrated that a Southern-style diet – high consumption of processed meats, fried foods and sugar-sweetened beverages – is associated with an increased risk of sepsis( Reference Gutiérrez, Judd and Voeks 9 ). Other studies have shown that a greater adherence to a Western diet – high consumption of red meats, fat/dairy productions and refined grains – is associated with higher levels of biomarkers of endothelial and inflammation dysfunction( Reference Hlebowicz, Persson and Gullberg 10 – Reference Oude Griep, Wang and Chan 12 ), which are both implicated in the biological pathway of sepsis( Reference Aird 13 – Reference Wang, Shapiro and Griffin 15 ). These two human studies show that a Southern and Western dietary pattern are associated with a higher risk of sepsis. Despite these findings, there is a lack of research on the association between the Mediterranean-style diet (Med-style diet) and risk of sepsis.
The concept of a Mediterranean diet originated in the Mediterranean basin after the second world war( 16 ). It is a social practice that is based on knowledge, traditions, fishing, conservation, preparation, as well as culture( 16 ). A traditional Mediterranean diet consists of a high consumption of plant foods, high olive oil intake as the primary source of monounsaturated fat, low saturated fat intake with limited meat and dairy products consumption, and moderate fish and alcohol intake( Reference Trichopoulou, Corella and Martinez-Gonzalez 17 ). Med-style diet has been reported as a model for healthy eating secondary to its contributions to a more favourable health status and overall quality of life( Reference Serra-Majem, Roman and Estruch 18 , Reference Willett, Sacks and Trichopoulou 19 ), as well as its beneficial roles in reducing CVD and chronic degenerative disease occurrence( Reference Serra-Majem, Roman and Estruch 18 , Reference de Lorgeril, Salen and Martin 20 ). One study concluded that lower adherence to a Med-style diet was associated with higher risk of stroke( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). In contrast, another study showed that higher adherence to a Med-style diet was associated with a 30 % risk reduction in CVD( Reference Martinez-Gonzalez, Salas-Salvado and Estruch 22 ) and lower incidence of major CVD events, breast cancer and diabetes( Reference Bloomfield, Koeller and Greer 23 ). Since Med-style diet is associated with decreased risk of stroke and prior stroke is associated with greater risk of sepsis( Reference Wang, Shapiro and Griffin 24 ), we would expect to observe a decrease in risk of sepsis within the high Med-style diet adherence group. In this study, we seek to examine the association between adherence to Med-style diet and risk of sepsis in community-dwelling participants of the REasons for Geographic and Racial Differences in Stroke (REGARDS) study.
Methods
Study design and participants
REGARDS is a national, prospective, longitudinal study that enrolled 30 239 individuals from January 2003 to October 2007. Briefly, this study investigated black–white and regional differences in stroke incidence with an oversampling of black and white individuals. This sampling method included 30 % of participants from the stroke belt (North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana and Arkansas), 20 % from the stroke buckle region (the coastal plain of North Carolina, South Carolina and Georgia), and 50 % from other regions in the continental USA. Participants were at least 45 years of age at baseline and contacted twice a year via telephone calls. Detailed descriptions of the REGARDS cohort are published elsewhere( Reference Fung, Rexrode and Mantzoros 25 – Reference Howard, Cushman and Pulley 28 ). The REGARDS-sepsis study identified participants with infection and potentially reversible risk factors for sepsis at the individual and community level. REGARDS participants with data anomalies or without plausible energy intakes/incomplete dietary data were excluded from the current analysis. The institutional review boards of all participating institutions approved the study and all participants provided written consent.
Measures
Primary exposure
We used the Block 98 FFQ (NutritionQuest) to estimate usual dietary intake over the past year. To calculate the Med-style diet score, nine food groups were identified: (i) vegetables, (ii) fruits, (iii) legumes, (iv) cereals (which includes bread, pasta and rice), (v) fish, (vi) meat, (vii) dairy products, (viii) fat intake and (ix) alcohol intake( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). Secondly, we regressed energy intake (kJ) to calculate the derived residuals of daily intake (g) for seven of the nine food groups (vegetables, fruits, legumes, cereals, fish, meat and dairy products)( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). Individuals received a value of 1 under these conditions: (i) for consumption above the median for each beneficial component (fruit, legumes, cereal, vegetables and fish) and (ii) for consumption below the median for each detrimental component (meat and dairy products)( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). For the eighth food category (fat intake), we used a ratio (monounsaturated fats to saturated fats) of the individual’s daily consumption to calculate the median consumption separately for each sex( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). Those individuals with a ratio equal to or above the median calculated received a score of 1( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). For alcohol intake, individuals with moderate consumption (1–7 drinks/week for women and 1–14 drinks/week for men) received a score of 1( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). The median intake of these components for males and females are shown in online Supplementary Table S1, which is based on Trichopolou’s proposed method( Reference Trichopoulou, Costacou and Bamia 29 ).
Primary outcome
Sepsis events were ascertained via medical record review from 5 February 2003 to 31 December 2012 and is described elsewhere( Reference Wang, Shapiro and Griffin 24 ). In brief, this process was conducted in strictly structured manner. Two independently trained research assistants collected and reviewed medical records for all hospitalisations and emergency department visits. Two abstractors independently reviewed all relevant clinical impressions documented in physician notes to confirm the presence of a serious infection during the initial hospital visit. Then these abstractors identified physiologic and laboratory parameters needed to fulfill the sepsis criteria below( Reference Levy, Fink and Marshall 30 ): Sepsis was defined as an admission to the hospital following serious infection and the existence of a minimum of two ‘systemic inflammatory response syndrome’ criteria during the first 28 h of hospital admission: (a) temperature (<36 or >38°C), (b) heart rate >90 beats per min, (c) respiratory rate >20 breaths/min or partial pressure of carbon dioxide (PaCO2) <32 mmHg and (d) leucocyte count (<4000 or >12 000 cells/mm3). When abstractors disagreed, additional physician-level review was utilised as needed.
Secondary outcome
Our secondary outcome was severe sepsis, which, due to advances in sepsis pathobiology( Reference Singer, Deutschman and Seymour 31 ), has been differentiated from less severe sepsis( Reference Singer, Deutschman and Seymour 31 ). We used the established sequential organ failure assessment (SOFA) criteria( Reference Vincent, de Mendonca and Cantraine 32 ) to identify admitted patients who had a SOFA score of at least two points, which we defined as a severe sepsis event. This score assesses organ dysfunction in six organ systems (respiration, coagulation, liver, cardiovascular, central nervous system and renal) and thresholds for SOFA score calculation are described elsewhere( Reference Vincent, Moreno and Takala 33 ).
Demographic and lifestyle factors
REGARDS used a combination of computer-assisted telephone interviews and a home visit follow-up roughly 3–4 weeks post interview to collect baseline data variable information. During the home visit, blood, urine, blood pressure, electrocardiogram, prescription medication and anthropometric data were collected. Participants were given self-administered questionnaires – including the FFQ – to gather information and return by mail. REGARDS collected information on age, sex, race, region of residence, income, education, smoking status, energy intake (kJ), sedentary behaviour and co-morbid conditions (see online Supplementary Table S2 for detailed description of each variable)( Reference Fung, Rexrode and Mantzoros 25 – Reference Howard, Cushman and Pulley 28 , Reference Sofi, Cesari and Abbate 34 ).
Statistical analysis
We used χ 2 and Kruskal–Wallis tests to compare socio-demographic characteristics across the distribution of Med-style diet scores. Individuals with implausible energy intakes or incomplete dietary data were excluded from analysis. The Med-style diet score was calculated as the sum of the nine food categories (range, 0–9), with a higher score indicating higher adherence to the Med-style diet. Then, we categorised Med-style diet score into low, moderate and high using Med-style diet score tertiles (Med-style diet score, 0–3, 4–5 and 6–9, respectively)( Reference Feart, Samieri and Rondeau 35 – Reference Tsivgoulis, Judd and Letter 37 ), which yielded comparable results to previous studies that evaluated Med-style diet with other health outcomes( Reference Tsivgoulis, Psaltopoulou and Wadley 21 , Reference Feart, Samieri and Rondeau 35 – Reference Tsivgoulis, Judd and Letter 37 ), with low Med-style diet group as the reference group, as previously described( Reference Tsivgoulis, Psaltopoulou and Wadley 21 ). We also compared included v. excluded participants. We performed a Cox proportional hazard model analysis adjusted for covariates, which included sociodemographic (age, race, sex, education, income and geographic region), lifestyle factors (smoking status and sedentary behaviour), and co-morbid conditions (diabetes, hypertension, stroke, atrial fibrillation and obesity status) to assess the association between Med-style diet and sepsis outcomes. We used the SOFA criteria to determine the association between Med-style diet and severe sepsis. Based on sepsis and severe sepsis definitions, sensitivity analyses were used to evaluate Med-style diet as a continuous variable. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
REGARDS collected information for 30 239 individuals. After excluding those with data anomalies (n 56) and with missing dietary data/implausible energy intakes (n 8927), a sample of 21 256 (70·3 %) individuals were included in the present analyses (Fig. 1). The median for age was 64·0 (interquartile range 13·0) years, 33·4 % were black (n 7099), 56·0 % were females (n 11 905) and 43·7 % were from the stroke-belt region (n 9286). The Med-style diet score ranged from 0 to 9 with an approximately normal distribution, with 26·0 % of participants (n 5519) having a score of 6–9, 42·1 % of participants (n 8940) having a score of 4 or 5 and 32·0 % of participants (n 6797) having a score of 0–3. The mean Med-style diet score was 4·4 (sd 1·7). Demographic characteristics and lifestyle factors of participants with low, moderate and high Med-style diet are presented in Table 1. Med-style diet score was greater among black race, male sex, older individuals, with greater educational attainment and residents of the non-belt, and was lower among current smokers, those who were obese and lived a sedentary lifestyle, had a lower energy intake, and had a history of diabetes, hypertension and stroke.
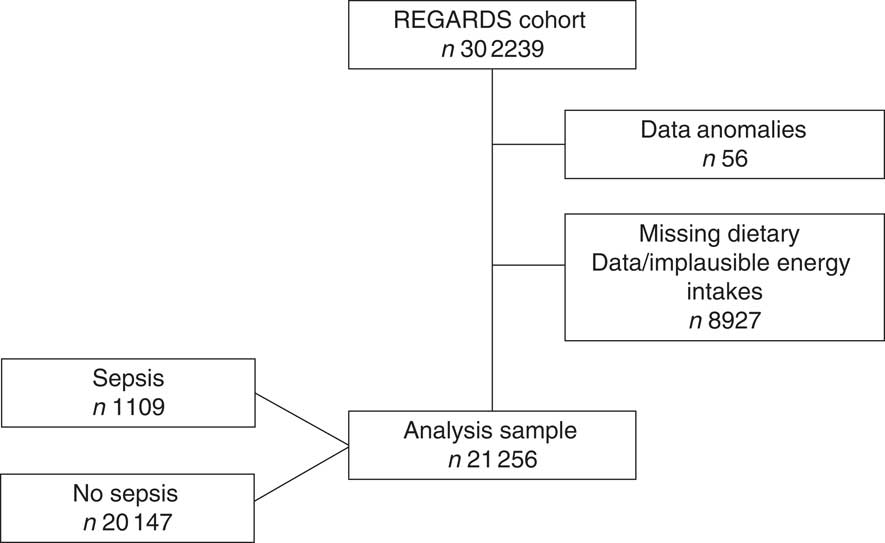
Fig. 1 Participant selection. REGARDS, REasons for Geographic and Racial Differences in Stroke.
Table 1 Description of baseline characteristics across tertiles of Mediterranean-style diet (Med-style diet) scores for included study participantsFootnote * (Numbers and percentages; medians and interquartile ranges (IQR))
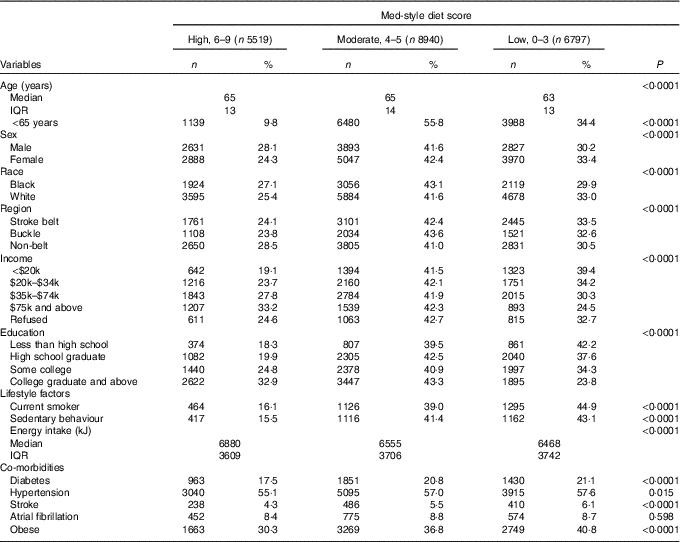
* Row percentages may not add to 100 due to rounding.
We did not observe differences in age or energy intake among individuals who were excluded v. included individuals. However, we did observe statistically significant differences for excluded individuals, who were more likely to be lower income, black males with less than high school education who resided in the non-belt region, were obese, lived a sedentary lifestyle, had history of diabetes and stroke, and were current smokers (not shown).
During a mean follow up period of 6·4 (sd 2·2) years, incident sepsis was identified in 1109 (5·2 %) participants. Results of our incremental Cox proportional hazard model analysis is shown in Table 2. As compared with low Med-style diet adherence, there were no statistically significant associations of moderate Med-style diet adherence with risk of sepsis in unadjusted or fully adjusted models (hazard ratio (HR) for moderate v. low Med-style diet=0·88; 95 % CI 0·77, 1·00; HR=0·93; 95 % CI 0·81, 1·08, respectively). Compared with low adherence to Med-style diet, higher adherence to Med-style diet was associated with lower risk of sepsis (HR for high v. low Med-style diet=0·66; 95 % CI 0·56, 0·78), in the unadjusted model. After adjustment for demographic and socioeconomic factors (model I, model II and model III), this association remained statistically significant (HR=0·62; 95 % CI 0·53, 0·74; HR=0·70; 95 % CI 0·59, 0·82 and HR=0·72; 95 % CI 0·60, 0·86, respectively). In the fully adjusted model (model IV), high adherence to Med-style diet remained associated with lower risk of sepsis (HR for high v. low Med-style diet=0·74; 95 % CI 0·61, 0·88).
Table 2 Sepsis according to tertiles of Mediterranean-style diet (MeD) scoreFootnote * (Hazard ratios (HR) and 95 % confidence intervals)

Ref., reference.
* Model I adjusts for age, race, sex and region. Model II adjusts for model I plus income and education. Model III adjusts for model II plus smoking status, sedentary behaviour and energy intake. Model IV adjusts for model II plus diabetes, hypertension, stroke and obesity status.
Moderate and high adherence to Med-style diet was associated with a lower risk of severe sepsis in the fully adjusted model (HR for moderate v. low Med-style diet=0·82; 95 % CI 0·68, 0·97 and HR for high v. low Med-style diet=0·65; 95 % CI 0·52, 0·81; respectively, table not shown). As a sensitivity analysis, Med-style diet score was evaluated as a continuous variable in a Cox proportional hazards model. In the fully adjusted model, a 1-point increase in Med-style diet score was independently associated with a 6 % lower risk of sepsis (95 % CI 3, 10). Independent of covariates in the fully adjusted model, a 1-point increase in Med-style diet score was associated with a 9 % lower risk of severe sepsis (95 % CI 5, 14).
Discussion
After adjusting for multiple potential confounders in the REGARDS cohort – a large population-based sample of US black and white adults, individuals within the high Med-style diet group had a 30 % lower risk of sepsis.
Med-style diet and risk of sepsis has been understudied; therefore, we are unable to compare our results to past studies. However, greater adherence to a Southern-style dietary pattern – high in eggs/egg dishes, sugar-sweetened beverages, fried foods, processed meats and red meat – has been associated with greater sepsis risk( Reference Gutiérrez, Judd and Voeks 9 ). This Southern-style pattern contains very different foods from those of the Med-style diet. Studies have also shown that Westernised diets are associated with some key mediators of sepsis, which includes inflammation and endothelial cell activation( Reference Hlebowicz, Persson and Gullberg 10 – Reference Oude Griep, Wang and Chan 12 ), whereas a healthier diet with high consumption of fruits and vegetables was associated with the opposite outcome( Reference Boynton, Neuhouser and Wener 38 , Reference Esposito, Marfella and Ciotola 39 ). Since the Med-style diet score is indicative of a healthy diet pattern in contrast to a Western or Southern diet pattern, our findings are congruent with those described above.
Our secondary analysis revealed a 18 % lower risk of severe sepsis for the moderate Med-style diet adherence group and a 35 % lower risk of severe sepsis for the high Med-style diet adherence group, in the fully adjusted model. Based on six organ systems, the SOFA score can capture organ dysfunction( Reference Singer, Deutschman and Seymour 31 ). A sepsis-SOFA score of 2 or more is associated with a 10 % excess in overall in-hospital mortality( Reference Singer, Deutschman and Seymour 31 ). Considering the vulnerability of this population and severity of organ dysfunction, we would expect to observe a potentially greater benefit of moderate as well as high adherence to Med-style diet.
There are some limitations to consider that can affect the generalisability of our findings. Reliance on FFQ data can have some methodological issues – selection bias – and underestimation of total energy intake, which can result in misclassification( Reference Willett 40 ). However, in our study, we examined participants based on a continuum of intakes of foods association with Med-style diet, which did not involve analysing the absolute energy intakes of each individual. Underestimation of food intake attenuates relative risk of a disease, which is secondary to nutrition literacy, education, socioeconomic status, availability of certain foods, and access to certain foods( Reference Kipnis, Subar and Midthune 41 – Reference Wedick, Ma and Olendzki 45 ). Secondly, the Med-style diet score was assessed using only baseline data. Therefore, we were not able to measure changes in diet at follow-up, which could potentially affect the reported associations. Generalisability of findings is limited because REGARDS includes only black and white individuals. However, the REGARDS cohort is a national cohort study that has an oversampling of blacks and whites from the USA stroke belt region, which is an area where sepsis rates are higher than the any other part of the USA( Reference Howard, Cushman and Pulley 28 ).
We acknowledged that all strategies of retrospective chart review are subjects to numerous forms of bias. However, identification of sepsis events was performed in a strictly structured manner. Initially, 1349 medical records were reviewed and excellent inter-rater agreement was shown for presence of serious infection (κ=0·92) and sepsis (κ=0·90)( Reference Wang, Shapiro and Griffin 24 ). Therefore, we believe that this system was actually more objective than expert clinician assessment, as it broke down the classification of sepsis to its component items (presence of serious infection plus at least two abnormalities in temperature, heart rate, respiratory rate or leucocyte count).
Patients were given instructions on how to complete their FFQ and mailed these questionnaires to the study centre, which resulted in available FFQ data for 70·4 % of participants. As mentioned earlier, there were no appreciable differences in age or energy intake among the excluded v. included participants; however, there were statistically significant differences in sex, race, region of residence, income, education, sedentary behaviour and smoking status, which is similar to other studies that examined REGARDS participants and Med-style diet( Reference Tsivgoulis, Psaltopoulou and Wadley 21 , Reference Tsivgoulis, Judd and Letter 37 ). The included participants were more likely to be white, female, have higher education and income. They were also less likely than excluded participants to be a smoker or to live a sedentary lifestyle. The included participants appear to have been healthier than the excluded participants. Participation rates among REGARDS participants favourably compare with other studies. In terms of bias, we would have to assume that the reason for an individual dropping out of a study could change the association between diet and disease process. Fortunately, for longitudinal cohort studies, most declines in participation rate are not likely to have a substantial impact on associations between the exposure and outcome( Reference Galea and Tracy 46 ). Therefore, we suggest that socio-demographic differences in participation rates unlikely affect the reported associations.
In conclusion, high adherence to Med-style diet was associated with a lower risk of sepsis. The moderate and high Med-style diet adherence group was associated with risk reduction of severe sepsis. Our findings suggest an association between Med-style diet and risk of sepsis. Targeted efforts to increase consumption of fruits, vegetables, legumes, fish and cereal with low consumption of meat, dairy products, fat, and moderate alcohol intake could potentially serve as an intervention for reducing risk of sepsis. A randomised control trial showed that a Med-style diet – supplemented with extra-virgin olive oil or nuts, along with high consumption of fruits, vegetables, legumes, white meat, fish and moderate alcohol intake – reduced the incidence of major cardiovascular events( Reference Estruch, Ros and Salas-Salvadó 47 ). This reiterates the potential benefits of a Med-style diet intervention in reducing risk of sepsis. To our knowledge, this is the first study to assess an association between Med-style diet and risk of sepsis as well as severe sepsis in community-dwelling participants. Therefore, future studies should further assess the potential benefits of a Med-style diet in reducing risk of sepsis using the SOFA criteria and assess any differences in Med-style diet over time.
Acknowledgements
The authors thank the investigators, the staff and participants of the REGARDS as well as REGARDS-sepsis studies for their valuable contributions.
This study was supported by the National Institute for Nursing Research (R01-NR012726), the National Center for Research Resources (UL1-RR025777), as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement (U01-NS041588) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. This work was supported by the National Institute of General Medical Sciences (J. P. D., F31-GM122180).
M. S. G., H. E. W. and S. E. J. designed research; H. E. W. and S. E. J. conducted research; M. S. G. and J. P. D. performed statistical analysis; M. S. G., H. E. W., K. D. M. and S. E. J. drafted and revised it critically for important intellectual content; M. S. G., H. E. W., K. D. M., S. E. J., O. M. G., J. P. D. and J. M. S. are responsible for final content of manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002866








