Introduction
Reintroductions of living organisms have become an important and frequently used tool in conservation management to help in the recovery, establishment or re-establishment of viable populations of threatened species (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Dodd and Seigel Reference Dodd and Seigel1991, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996, Fischer and Lindenmayer Reference Fischer and Lindenmayer2000). In recent decades, a large number of reintroduction programmes have been developed worldwide, resulting in some successes but many failures (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Dodd and Seigel Reference Dodd and Seigel1991, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996, Fischer and Lindenmayer Reference Fischer and Lindenmayer2000). Therefore, monitoring is critical to evaluate the outcome of reintroduction programmes and to determine the precise factors that underlie their success or failure.
Predictive models are being increasingly used in reintroduction programmes both to identify suitable locations (e.g. Pearce and Lindenmayer Reference Pearce and Lindenmayer1998, Thatcher et al. Reference Thatcher, Van Manen and Clark2006, Olsson and Rogers Reference Olsson and Rogers2009), and to predict population growth and range expansions of released species (e.g. Carroll et al. Reference Carroll, Phillips, Schumaker and Smith2003, Kramer-Schadt et al. Reference Kramer-Schadt, Revilla, Wiegand and Breitenmoser2005). While factors such as stochastic mortality or sex ratio are important to success during the establishment phase, habitat factors are most likely to determine the spread of the reintroduced population once it has been established, (Bright and Smithson Reference Bright and Smithson2001, Le Gouar et al. Reference Le Gouar, Robert, Choisy, Henriquet, Lecuyer, Tessier and Sarrazin2008). After the establishment phase, information collected during the first years of the reintroduction programme may be used to refine potential habitat models as well as to predict population development (South et al. Reference South, Rushton and Macdonald2000). Monitoring may allow the design of suitable management actions and thus the development of an adaptive management strategy that is essential in reintroduction programmes (Sarrazin and Barbault Reference Sarrazin and Barbault1996).
Lesser Kestrel Falco naumanni is a small colonial bird of prey typically inhabiting Palearctic pseudo-steppes during the breeding season. This species nests in crevices or cavities of farm buildings, old churches or castles (Cramp and Simmons Reference Cramp and Simmons1980). Lesser Kestrel was once one of the most abundant birds of prey in Europe but underwent a large population decline during the second half of the 20th century (Biber Reference Biber1990). Since the 1990s, populations are recovering in Spain and southern France (e.g. Prugnolle et al. Reference Prugnolle, Pilard, Brun and Tavecchia2003, Ortego et al. Reference Ortego, Aparicio, Calabuig and Cordero2007). As in other European countries, breeding populations in Spain suffered large population declines between 1960 and 1980 (Tucker and Heath Reference Tucker and Heath1994). Consequently, several reintroduction programmes were established (e.g. Pomarol Reference Pomarol, Nicholls and Clarke1991, Serrano and Delgado Reference Serrano and Delgado2004, this study). In eastern Spain, the species was wiped out at the beginning of the 1990s and the local government began a reintroduction programme in 1997 (Alberdi Reference Alberdi, Garcés and Corroto2001). Between 1997 and 2001, 184 chicks from the west of Spain were released in a small agricultural valley (10,000 ha; 38°94’N, 01°11’W) with adequate foraging habitat (traditionally-farmed cereals with field margins and small patches of natural vegetation) (Donázar et al. Reference Donázar, Negro and Hiraldo1993, Tella et al. Reference Tella, Forero, Hiraldo and Donázar1998, Tella and Forero Reference Tella and Forero2000, Franco and Sutherland Reference Franco and Sutherland2004). According to the hacking protocol (Sherrod et al. Reference Sherrod, Heinrich, Burnham, Barclay and Cade1982), kestrel chicks were placed in hack boxes at the reintroduction site at 20–24 days old, and after a few days of acclimatisation, boxes were opened and the birds were allowed to leave. The reintroduction programme was successful and this new colony is now established. Ten years after the reintroduction programme, it is important to assess whether the availability of nest sites may limit the expansion (numerical and spatial) of the newly established colony. The shortage of suitable nesting sites is a key factor limiting population size in many hole-nesting birds (Village Reference Village1983, Newton Reference Newton1994, Reference Newton1998), although it has been identified as important in only some populations of Lesser Kestrels (Forero et al. Reference Forero, Tella, Donázar and Hiraldo1996, Franco et al. Reference Franco, Marques and Sutherland2005). However, the availability of suitable nest-sites can be crucial for reintroduced populations of Lesser Kestrels if this factor is not adequately foreseen. In our case study, only 10 nest-boxes were provided in the hacking area, thus Lesser Kestrels have to nest on isolated buildings in the reintroduction area, although no previous assessment of the suitability of the buildings for nesting was carried out. Other authors have studied the characteristics and availability of buildings for the ‘natural’ expansion of colonies by comparing the buildings with and without colonies, and concluded that Lesser Kestrels selected buildings with many roof and wall cavities that are surrounded by extensive cereal and fallow fields (Franco et al. Reference Franco, Marques and Sutherland2005). We use the information collected during the monitoring of the reintroduction programme of Lesser Kestrels in eastern Spain to determine the characteristics of buildings that make them suitable for reintroduced Lesser Kestrels. We use this information to predict the availability of nesting sites for the population growth and based on these results, we make recommendations for conservation management.
Methods
Field methods and habitat variables
During the 2007 breeding season (from the end of February to the beginning of July), we surveyed all buildings in an area within a maximum radius of 7,700 m around the reintroduction site to determine whether they were occupied by Lesser Kestrels and the number of breeding pairs per occupied building. This spatial limit is the median distance covered by dispersing Lesser Kestrels (Serrano et al. Reference Serrano, Tella, Forero and Donázar2001, Serrano et al. Reference Serrano, Tella, Donázar and Pomarol2003). Buildings were considered to be occupied if one or more pairs were seen entering a cavity and when a male brought food to feed a female (Ursúa et al. Reference Ursúa, Tella, Serrano, Seoane, Gajón and Forero2004a).
To identify the characteristics of buildings that make them suitable for Lesser Kestrels, we surveyed both occupied and non-occupied buildings in the study area (n = 76). The mean distance between the hacking site and the buildings included in the study area was 3,600 m (range = 0–7,700 m; SE = 1,808 m). Buildings were characterised according to 11 variables (Table 1). The percentage of suitable foraging habitat around each building was obtained in a previous study in the reintroduction area (authors’ unpublished data) by means of the Savage Selectivity Index (Manly et al. Reference Manly, McDonald and Thomas1993). This index is expressed as ωi = Ui / pi, where Ui is the proportion of observations recorded in any one land-use and pi is the proportion of that land-use in the study area. Our results, which agree with other studies (Donázar et al. Reference Donázar, Negro and Hiraldo1993, Tella et al. Reference Tella, Forero, Hiraldo and Donázar1998, Tella and Forero Reference Tella and Forero2000), showed that Lesser Kestrels selected fallow land, stubble and cereals as foraging habitat.
Table 1. Independent variables analysed for suitability of isolated houses/buildings for Lesser Kestrels.

Statistical analysis
To relate the occupancy and abundance of Lesser Kestrels with building characteristics, we used logistic regression models with a binomial error and a logistic link function for our binary dependent variable (occupied/unoccupied building) and Poisson regression models with a Poisson error and a logarithm function for our count dependent variable (number of breeding pairs per building). We developed univariate and multivariate models between the predictor variables and both dependent variables. Univariate models illustrate the relationship between the presence and abundance of the species and each of the predictor variables independently. Multivariate models consider the influence and co-linearity of multiple factors by jointly modelling multiple response variables. The result is a model for each one of the dependent variables with the maximum number of significant response variables and with the larger explained deviance. The resulting multivariate models were used to predict the availability of Lesser Kestrel nest-sites in the reintroduction area. The inclusion of variables into the multivariate models was made step-by-step using Akaike’s information criterion (AIC) (Burnham and Anderson Reference Burnham and Anderson2002). However, to increase the parsimony of our multivariate models, variables were added only if they resulted in a > 1% increase in the explained deviance. The linear and quadratic forms of all explanatory variables were tested.
We used the probability value that maximised the sum of sensitivity and specificity (Liu et al. Reference Liu, Berry, Dawson and Pearson2005) as the threshold value of occurrence for the models. Model values were transformed from quantitative values to a qualitative range of habitat quality and abundance. In the transformations of both models, the first category included all values ranging from 0 to the threshold value of occurrence, which thus represented the characteristics of suboptimal buildings in which the species is not expected to nest. The remaining values were proportionally grouped in equal intervals that represent classes of buildings with different quality for the nesting of the species.
We used the AUC values (area under the receiver operating characteristic [ROC] curve) to assess the predictive ability of the logistic model (Fielding and Bell Reference Fielding and Bell1997). The Poisson model was evaluated using Pearson and Spearman coefficients. All analyses were conducted using the R statistical package (R Development Core Team 2008).
Results
In 2007, 20 buildings were occupied (26.32% of the total number of buildings surveyed) by 40 Lesser Kestrel pairs, with a mean abundance of 2.4 pairs per building (range = 1–5; SE = 1.6).
Univariate models
The chances of a building being occupied by Lesser Kestrels in the study area increased with suitable foraging habitat around the colony (FORAG) and with the building basal area (AREA), to an optimum of c.150 m, but decreased with the presence of trees (TREE) (Table 2, Figure 1). The probability of being occupied decreased to almost 0 when more than 50% of the perimeter of the building had trees (Figure 1).
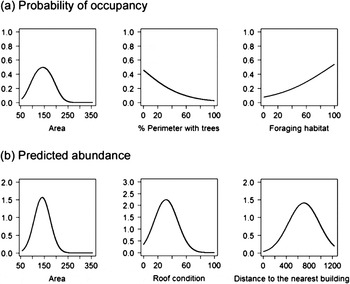
Figure 1. Probability of occupancy (a) of Lesser Kestrels in relation to the independent variables AREA (left), TREE (centre), and FORAG (right); and predicted abundance (pairs per building) (b) in relation to AREA (left), CON (centre), and DINHA (right). See Table 1 for an explanation of the independent variables.
Table 2. Univariate GLM that relates occupation and abundance of the Lesser Kestrel with the characteristics of buildings. R 2 = % of explained deviance. Type = type of response with the larger value of explained variance: “+” linear positive, “−“ linear negative, “++” quadratic positive. P = * < 0.05, ** < 0.01, *** < 0.001.

The results obtained in the abundance model (Table 2) predicted that Lesser Kestrel abundance was highest in buildings with a basal area of c.150 m2, with a distance of c.800 m to the nearest inhabited building (DINHA) and with c.25% of the roof in ruins (CON) (Figure 1). The predicted abundance decreased to almost 0 when more than 50% of the roof was in ruins and when more than 50% of the perimeter had trees (Figure 1). The abundance of kestrels increased linearly with suitable foraging habitat around the colony (FORAG).
Multivariate models
The predictive occupancy model incorporated the variables AREA and TREE and explained 23.85% of the deviance (Table 3). The threshold value of occurrence was 0.35 (Table 4). Table 4 shows the grouping of the buildings within the classes of probability of occupation by Lesser Kestrels.
Table 3. Multivariate GLM for the occupation and abundance of Lesser Kestrels in the reintroduction area.

Table 4. Qualitative classes of the presence and abundance models. The number of the observed and predicted buildings in each class is given. In the observed buildings row, suboptimal and optimal rows means buildings with presence and absence of Lesser Kestrels respectively.

Like the predictive occupancy model, the abundance model incorporated the variables AREA and TREE. In addition, this model incorporated the variables CON, DINHA, and the maximum height of the highest roof (MH) (Table 3). The resulting model explained 58.14% of the deviance. According to the predictions of the model, 10.53% of the buildings could have a higher number of Lesser Kestrel breeding pairs than they actually had (Table 4).
Taking into account the predictions of both multivariate models, 10 unoccupied buildings in the study area had a medium to high probability of being occupied by Lesser Kestrels and so the population could increase by eight new breeding pairs.
The evaluation of the model of occupancy showed AUC values of 0.8232. The relationship between predicted abundance and current abundance of Lesser Kestrels was statistically significant (P < 0.01) and positive with Pearson and Spearman correlation coefficients of 0.7272 and 0.5519, respectively.
Discussion
Building selection
According to our results, Lesser Kestrels reintroduced in eastern Spain selected medium-sized buildings as nesting sites, with extensive land use in the area surrounding the colony site and with few or no trees. In addition, Lesser Kestrel abundance was explained by roof condition and distance to the nearest occupied building. The preference of Lesser Kestrels for extensive land use around nest-sites has also been found by other authors both on a regional scale (Bustamante Reference Bustamante1997) and at a colony level (Franco et al. Reference Franco, Marques and Sutherland2005). This preference is likely to be linked to foraging habitat because Lesser Kestrels forage in extensive fields where prey items are abundant (Tella et al. Reference Tella, Forero, Hiraldo and Donázar1998, Ursúa et al. Reference Ursúa, Serrano and Tella2004b, Rodríguez et al. Reference Rodríguez, Johst and Bustamante2006). Building size and roof condition may be related to the availability of cavities for nesting, i.e. good roof condition and small buildings decrease the availability of cavities for nesting. Other authors have also detected the preference of Lesser Kestrels for buildings with many roof and wall cavities (Franco et al. Reference Franco, Marques and Sutherland2005). The avoidance of large buildings does not have a clear explanation; this response may be masking the correlation of this variable with other explanatory factors, e.g. in our study area most of the largest buildings did not have a roof with an adequate design for the nesting of the species. In relation to the preference of buildings without trees, this response may be linked to the avoidance of predators, such as domestic cat Felis catus, Black Rat Rattus rattus, and Stone Marten Martes foina (Negro and Hiraldo Reference Negro and Hiraldo1993). Other studies have concluded that Lesser Kestrels avoid predation by selecting the highest positions in farmhouse roofs (Negro and Hiraldo Reference Negro and Hiraldo1993). In our case study, height of building was not important as it was not included in the list of significant variables in the univariate models but did appear in the abundance multivariate model. Finally, the selection of buildings located at a moderate distance from other unoccupied buildings shows the preference of Lesser Kestrels for rural landscapes with scattered buildings.
Among the factors detected that determined the presence and abundance of Lesser Kestrels, surrounding habitat and roof condition are of special concern. On one hand, habitat loss, mainly due to agricultural intensification, is the main threat to the conservation of the Lesser Kestrel (Donázar et al. Reference Donázar, Negro and Hiraldo1993, Parr et al. Reference Parr, Collin, Silk, Wilbraham, Williams and Yarar1995, Bustamante Reference Bustamante1997, Tella et al. Reference Tella, Forero, Hiraldo and Donázar1998, Tella and Forero Reference Tella and Forero2000, Franco and Sutherland Reference Franco and Sutherland2004). Currently in the study area, traditional extensive agriculture (cereal plantations) is being transformed into unsuitable foraging habitat for the species (vine, sunflower plantations and fruit trees) and other unproductive habitats (urbanisation and installation of solar farms). This change in land use leads to a lowering of habitat quality and thus it is predicted that it will prevent population growth. On the other hand, in other colonies in the interior of the Iberian Peninsula, building condition is one of the threats to the species due to the possibility of collapse of roofs or whole buildings (Tella et al. Reference Tella, Pomarol, Muñoz and López1993, Franco et al. Reference Franco, Marques and Sutherland2005). However, in the study area as well as in other coastal areas in the Mediterranean, the problem of building condition is more related to building and roof rehabilitation with unsuitable materials and designs. In fact, after this study, some roofs in the study area were rehabilitated and prevented the Lesser Kestrels from nesting.
Nest site availability
Multivariate models predicted that the availability of adequate buildings for nesting as well as nesting sites within buildings may limit the population growth of the reintroduced population studied. Almost half of the buildings in the reintroduction area were predicted to be unsuitable for nesting by Lesser Kestrels and the reintroduced population may show only slight population growth, with a total of 48 predicted breeding pairs, and with most of the buildings containing a low number of breeding pairs (< 3). However, it has to be noted that a potential problem with our models is that they are based on data from an expanding population. Thus, currently unoccupied buildings may either be unsuitable for nesting or, if the population is not at carrying capacity, as the population increases birds may start to occupy these other buildings once the best ones are fully occupied. This would lead to an underestimate of the quality of buildings and thus of the population growth of the reintroduced population. Thus, it would be advisable to update the results of this study in order to validate the models and predictions obtained.
Despite this, the size of the reintroduced population and colonies seem to be smaller than what would be expected after 10 years since reintroduction and there may be several causes. The expansion of Lesser Kestrel colonies is often through despotic behaviour (Serrano and Tella Reference Serrano and Tella2007) which changes the spatial distribution of colonies by increasing the frequency of small colony sizes over large ones (> 10 breeding pairs) (Jovani et al. Reference Jovani, Serrano, Ursúa and Tella2008). Accordingly, the fact that all our colonies were small, with < 5 breeding pairs per colony, may be indicating that the buildings in the reintroduction area do not have enough nest sites of suitable quality.
An Allee effect (Serrano et al. Reference Serrano, Oro, Ursúa and Tella2005) and conspecific attraction (Serrano et al. Reference Serrano, Tella, Forero and Donázar2001, Reference Serrano, Tella, Donázar and Pomarol2003, Reference Serrano, Forero, Donázar and Tella2004, Serrano and Tella Reference Serrano and Tella2003) may also be slowing the growth of this reintroduced population. These processes may cause the migration of the breeding birds from the reintroduction site to other larger colonies, independently of the quality of the habitat of the natal colony. In an area c.25 km around the reintroduction area there are other dense populations of Lesser Kestrels (e.g. Núñez et al. Reference Núñez, Vélaz and Catalán2009) that may attract reintroduced kestrels. Although most individual Lesser Kestrels tend to settle close to their natal colony, juveniles may move large distances (> 100 km) (Serrano et al. Reference Serrano, Tella, Donázar and Pomarol2003) with a frequency higher than would be expected in a philopatric species (Alcaide et al. Reference Alcaide, Serrano, Tella and Negro2009). Future studies should try to elucidate the influence of these processes on the population growth of this reintroduced population. This may improve the predictions of population growth made in this study.
Management recommendations
According to our results, the limits for the growth of this reintroduced population seem to be related to the quality of the buildings, but also to the dispersal behaviour of the species. The relatively low population size of this reintroduced colony, and its low expected growth, could inhibit its long-term viability. Improving the quality of buildings may facilitate population growth. To this end, in buildings where other conditions are adequate (i.e. absence of trees and presence of extensive agriculture), nest boxes and special tiles could be installed. These measures have been successfully developed in other Iberian colonies of Lesser Kestrels (Pomarol Reference Pomarol1996, Franco et al. Reference Franco, Marques and Sutherland2005, Catry et al. Reference Catry, Alcazar, Franco and Sutherland2009) and in colonies of other small falcons (Hamerstrom et al. Reference Hamerstrom, Hamerstrom and Hart1973, Fargallo et al. Reference Fargallo, Blanco, Potti and Viñuela2001). Also, in areas with suitable habitat but without buildings, breeding towers may be constructed. In addition, special attention must be given when restoring old farmhouses to avoid the destruction of possible nesting cavities. Measures of rural development and habitat protection could be important to restrict the loss of suitable foraging habitat for the species. These measures may also help other threatened species in the reintroduction area, such as steppe birds (e.g. Little Bustard Tetrax tetrax, Pin-tailed Sandgrouse Pterocles alchata and Black-bellied Sandgrouse P. orientalis) (Urios et al. Reference Urios, Escobar, Pardo and Gómez1991).
However, considering the strong emigration of Lesser Kestrels from small to large relatively close colonies (Serrano et al. Reference Serrano, Oro, Ursúa and Tella2005), the utility of reintroducing colonies in areas close to other natural long-established population, as our case study, is highly doubtful. Furthermore, the reintroduction project in the study area aims to restore a locally extinct population of Lesser Kestrels while the main colonies of this species in the interior of the Iberian Peninsula continue to be threatened by habitat loss through agricultural intensification (Tella et al. Reference Tella, Forero, Hiraldo and Donázar1998, Tella and Forero Reference Tella and Forero2000). In a general strategy for the conservation of Lesser Kestrels, conservation of the main colonies is the priority rather than dedicating human and economic resources on manipulative actions for the establishment of new colonies. Unfortunately, many reintroduction programmes are based on local policies designed and implemented by local authorities, with a limited understanding of the status and conservation needs of the species at an ecological and ecosystem scale and no consideration of the metapopulation dynamics of the populations. This analysis continues the open debate about the true need for some reintroduction programmes and the importance of having a global vision of the species status and conservation (Frazer Reference Frazer1992, Meffe Reference Meffe1992).
Acknowledgements
This research was funded by ENESTAR S.A. We wish to thank José Daniel Anadón for his assistance in data analysis. He and José Antonio Sánchez-Zapata made useful comments on an earlier version of the manuscript. We are indebted to the referees of this manuscript for helpful comments that have much improved the paper. Mercedes Alberdi, Miguel Blázquez and Juan Manuel Pérez-García helped with fieldwork. We also thank all the inhabitants of the study area for their support during fieldwork.









