Implication
Sow productivity has increased dramatically in the last decade. With improved productivity, requirements for energy and amino acids increase during lactation. To meet these needs, sows should be in proper body condition before farrowing to encourage high feed intake, and provided full access to feed in the few days before and during lactation. Diets should contain high-energy, low-fibre ingredients to maximise energy intake, and formulated with sufficient amino acid levels to meet the demands for milk production and minimise tissue catabolism. Today’s sows are resilient and, with proper nutrient intake, can withstand the rigorous demands of increased productivity.
Introduction
Genetic selection and improvements in health, management and nutrition have led to unprecedented levels of sow productivity. In 2016, pigs weaned per sow per year averaged 25.7 in the United States, with an even higher productivity in the major pork production countries in Europe, ranging from 27.0 in Spain to 32.1 in Denmark (Agriculture and Horticulture Development Board, 2017).
Much of the increase in pigs weaned per sow has been a result of increased litter size. An increased use of genomics has accelerated the rate of progress in recent years. Data from Genus PIC illustrate the speed of change. From 2006 to 2019, the genetic trend at the nucleus level for total pigs born increased by approximately 0.334 pig per year, or an increase by 4.5 pigs per litter over the 13-year period (Figure 1). Initially, this led to a decrease in individual pig birth weight with average birth weight decreasing by approximately 120 g from 2006 to 2013 with a concomitant increase in pre-weaning mortality (Figure 2). After changing the selection criteria to offset this trend in 2013, the decrease was quickly reversed. Within 6 years, the previous loss in average birth weight was recovered, and in fact, the average birth weight was 20 g greater in 2019 than reported in 2006 while maintaining a steady increase in total born per litter. Because of heavier birth weights, pre-weaning mortality also decreased almost 6 percentage units from the high in 2013.
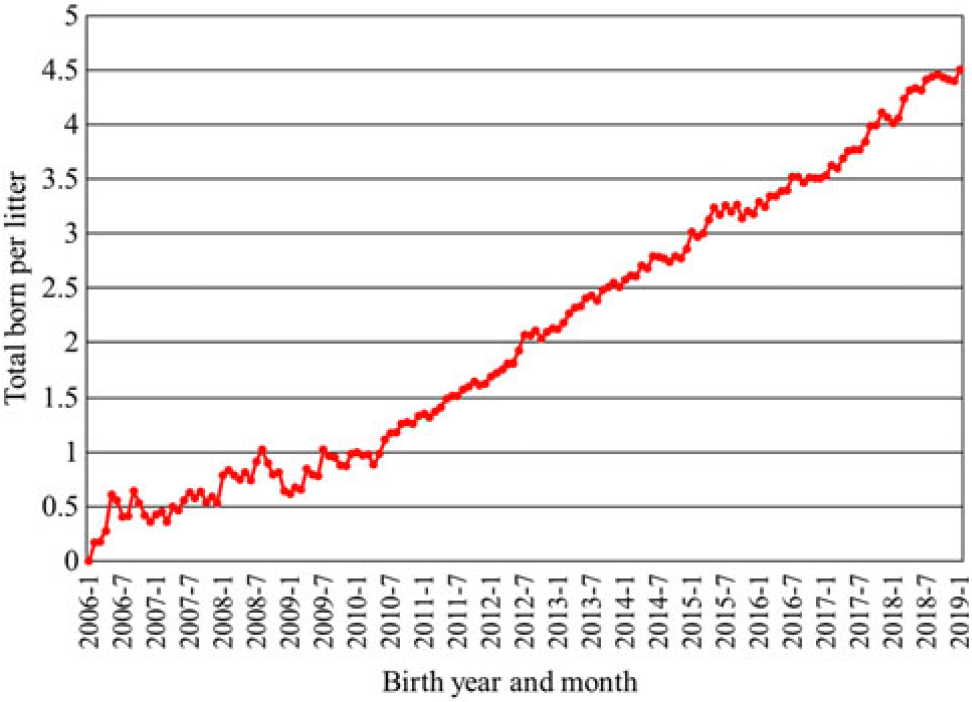
Figure 1 Genetic trend for total pigs born per litter at the nucleus level from Genus PIC (M. Culbertson, personal communications, 12 February 2019).
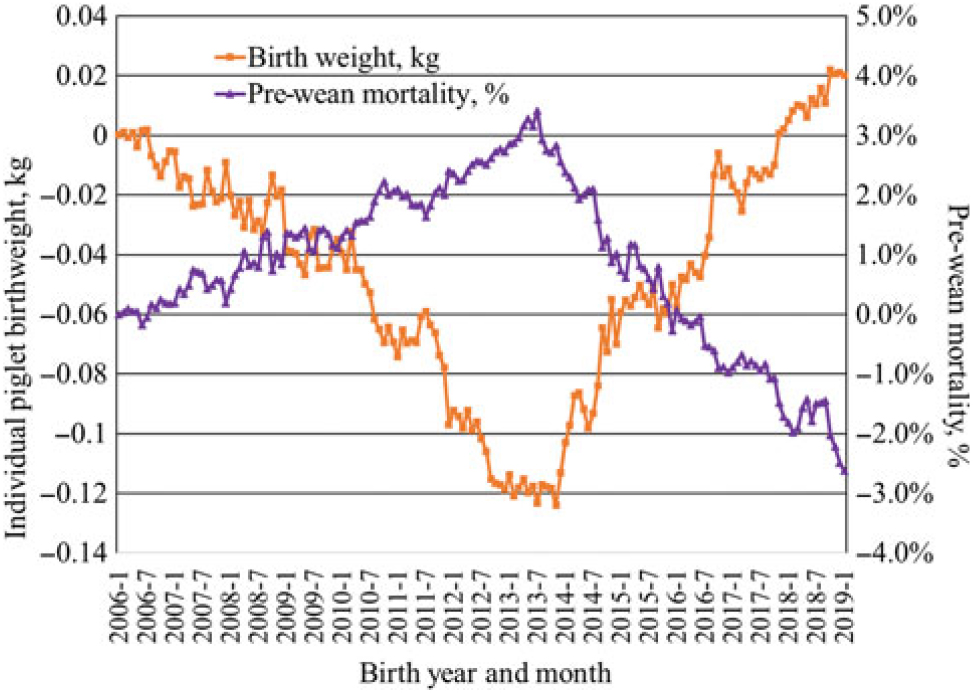
Figure 2 Genetic trend for individual pig birthweight and pre-wean mortality from Genus PIC (M. Culbertson, personal communications, 12 February 2019).
The improvements in reproductive performance increase metabolic demands on the sow during gestation and lactation. Today’s modern genotype females are also faster-growing and have less adipose tissue than their predecessors. In commercial production, it is not uncommon to see gilt tenth rib fat depth at farrowing average 16 mm and parity 2 and older sows having fat depth ranging from 12 to 16 mm (Kim et al., Reference Kim, Yang, Pangeni and Baidoo2015; Thomas et al., Reference Thomas, Goodband, Tokach, Woodworth, DeRouchey and Dritz2018). These changes in body composition and reproductive performance alter nutrient requirements during gestation and lactation. Increases in litter size increase total fetal growth in late gestation, farrowing duration, colostrum needs and milk production. In this review, the nutrient demands for these biological processes are discussed, dividing the sections into the peripartum and lactation periods and the unique requirements during each period.
Peripartum transition period
While several studies have been conducted to evaluate changing nutrient requirements in late gestation (day 90 to parturition), few studies have focused on the days immediately prior to parturition. The transition period has been loosely defined as the last 10 days of gestation to the first 10 days of lactation (Theil, Reference Theil and Farmer2015). During the peripartum transition period, a rapid shift in nutrient requirements and nutrient partitioning occurs due to an exponential increase in fetal and mammary growth, uterine components and colostrum synthesis (Feyera and Theil, Reference Feyera and Theil2017). Typically, sows are limit-fed a gestation diet, then receive a set amount of lactation feed for 2 to 3 days prior to farrowing. The lactation diet is a higher lysine (Lys), higher energy diet than the gestation diet. The change from lower Lys limit-fed gestation diet to a nutrient-dense lactation diet can be met with metabolic challenges as the sow has to rapidly adapt to a new diet composition. It is important to minimise this rapid shift in nutrients at the time of parturition to avoid a negative impact on parturition and lactation performance (Martineau et al., Reference Martineau, Le Treut, Guillou and Waret-Szkuta2013). The goal of the transition period should be to meet the changing requirements for fetal and mammary tissue growth, prepare the sow for the upcoming lactation demand and supply nutrients during parturition for maximum piglet survival at birth. Another critical activity in the peripartum transition period is colostrum production, which is estimated to begin 2 to 3 days before the onset of parturition (Devillers et al., Reference Devillers, Farmer, Mounier, Le Dividich and Prunier2004).
Transition period feeding and farrowing duration
Parturition is an energy-demanding process. As litter size continues to increase, there is also an increase in farrowing duration. A normal birthing interval is 15 to 20 min, which could lead to a 300-min farrowing duration for a litter of 15 piglets. Several factors have been associated with an increase in farrowing duration, including sow backfat >17 mm at farrowing (Oliviero et al., Reference Oliviero, Heinonen, Valros and Peltoniemi2010) and increased litter size (van Dijk et al., Reference van Dijk, van Rens, van der Lende and Taverne2005). Recently, Feyera et al. Reference Feyera, Pedersen, Krogh, Foldager and Theil(2018) observed that farrowing duration is reduced if sows have access to feed and eat at least 3 h before farrowing, hypothesising that this is due to a greater availability of energy. However, Cools et al. Reference Cools, Maes, Decaluwe, Buyse, van Kempen, Liesegang and Janssens(2014) fed a lactation diet ad libitum starting on day 105 of gestation and did not affect farrowing duration. This study had fewer total born (11 pigs), which may explain the reason why no differences were observed. Several other nutritional strategies during the transition period have been investigated for their effects on farrowing duration. Reduced farrowing duration was observed with added phytase (Manu et al., Reference Manu, Pangeni, Wilcock and Baidoo2018) or soluble fibre sources (Theil et al., Reference Theil, Flummer, Hurley, Kristensen, Labouriau and Sorensen2014), but not with creatine (Vallet et al., Reference Vallet, Miles and Rempel2013) or a dietary nitrate supplement (van den Bosch et al., Reference van den Bosch, Wijnen, van de Linde, van Wesel, Melchior, Kemp, van den Brand and Clouard2019). Interestingly, Feyera et al. Reference Feyera, Pedersen, Krogh, Foldager and Theil(2018) observed that during late gestation the uterus partially satisfies its energy demand using acetate and butyrate from dietary fibre inclusion. Conversely, during farrowing, these short-chain fatty acids are not extracted by the uterus, but rather triglycerides and glucose are used as the energy source. Therefore, while short-chain fatty acids may be used by the uterus in late gestation, feeding a diet containing increased triglycerides and glucose a day prior to parturition could supply the readily absorbed energy required by the uterus during parturition, which could positively benefit uterine contractions and reduce farrowing duration and stillbirth rate.
Energy requirements in the peripartum transition period
Dietary energy requirements during gestation are derived from body maintenance, growth of conceptus and maternal demands from the mammary and uterus. These requirements will also depend on sow BW, parity and environmental conditions (Trottier et al., Reference Trottier, Johnson, de Lange and Farmer2014). Of particular interest in the transition period are the requirements to support an exponential growth rate of the fetal, mammary and uterine components. Feyera and Theil (Reference Feyera and Theil2017) used a factorial approach to model metabolisable energy (ME) requirement in the last 12 days of gestation, and estimated a 60% increase in requirement during this time period from 33.9 to 55.6 MJ ME per day (Figure 3). The greatest proportion of required ME (75% to 80%) during the end of gestation is derived from maintenance and depends on sow BW gain (Noblet et al., Reference Noblet, Dourmad and Etienne1990). Thomas et al. Reference Thomas, Goodband, Tokach, Woodworth, DeRouchey and Dritz(2018) observed gilt-mobilised fat tissue to meet the energy needed in late gestation for fetal growth and colostrum production. Decaluwe et al. (Reference Decaluwe, Maes, Cools, Wuyts, De Smet, Marescau, De Deyn and Janssens2014) observed an increase in backfat loss from day 108 to farrowing when sows were only fed 1.5 v. 3.0 kg/day of a transition diet. Similarly, Cools et al. Reference Cools, Maes, Decaluwe, Buyse, van Kempen, Liesegang and Janssens(2014) observed that sows fed a lactation diet ad libitum from day 105 of gestation had less backfat thickness loss compared with limit-fed sows. Hansen et al. Reference Hansen, Lauridsen, Sørensen, Bach Knudsen and Theil(2012) observed that total intake of ME from day 108 to 112 of gestation was negatively correlated with piglet weight gain at peak lactation, indicating that a less negative energy balance around parturition is inhibitory for sow milk yield at peak lactation, likely because of the negative impact on feed intake. While energy supply in the peripartum transition period is important to meet changing tissue needs, it is crucial to supply energy without contributing to excess BW gain and backfat stores that will lead to a negative impact in lactation feed intake, milking ability and litter growth.
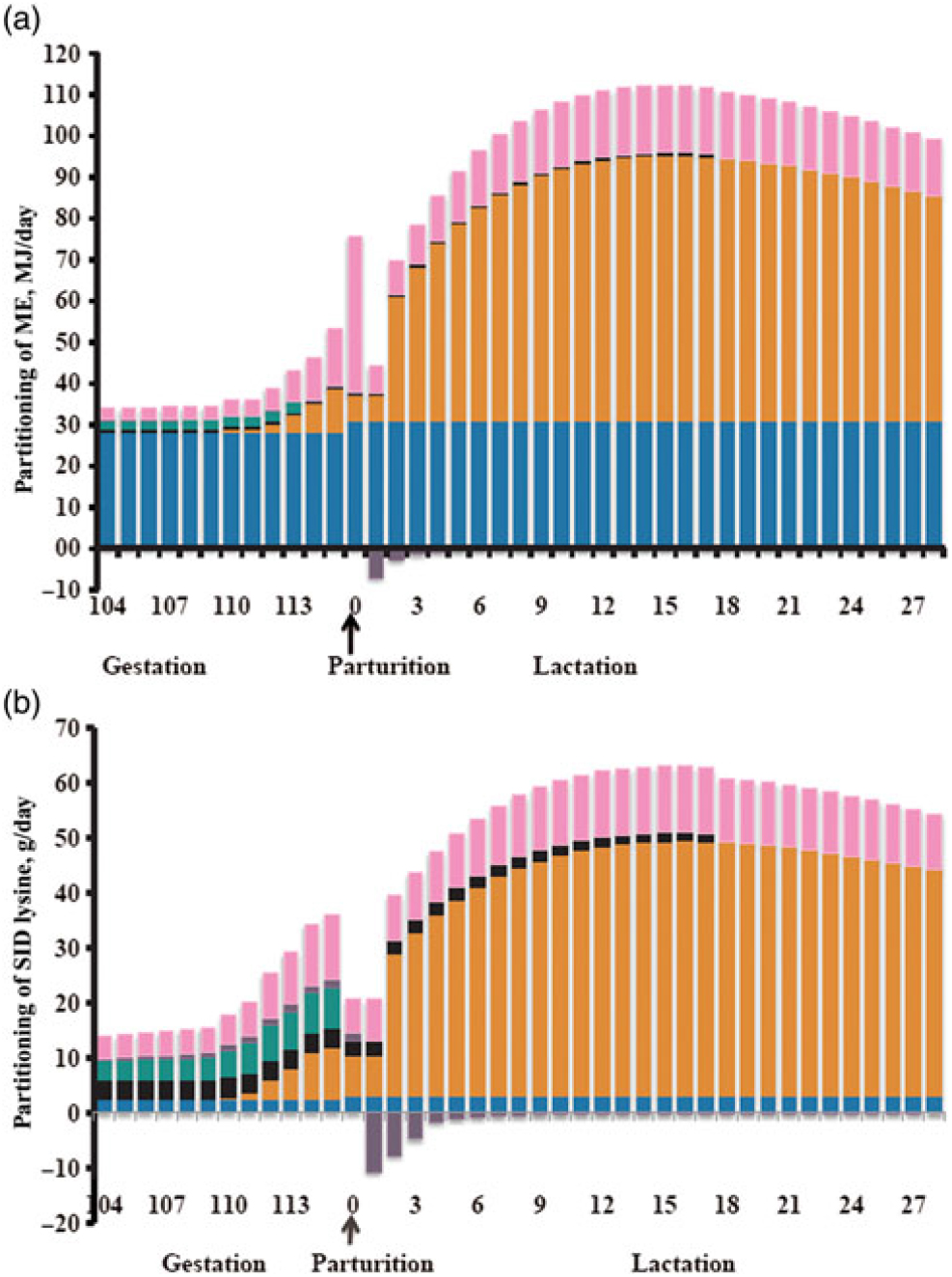
Figure 3 Calculated metabolisable energy (ME; panel a) and standardized ileal digestible (SID) lysine (panel b) requirements for maintenance (blue bars), colostrum/milk production (orange bars), mammary growth (black bars), fetal growth (green bars), uterine components (purple bars) and additional heat loss for energy or oxidation/transamination or amino acids (pink bars) in sows during transition and lactation. (Reprinted from Livestock Science, 201, Feyera and Theil, Energy and lysine requirements and balances of sows during transition and lactation: A factorial approach, 50–57, 2017, with permission from Elsevier.)
Colostrum intake is highly correlated with increasing piglet survivability, with a recommended intake of 200 ml per pig in the first 24 h (Ferrari et al., Reference Ferrari, Sbardella, Bernardi, Coutinho, Vaz, Wentz and Bortolozzo2014; Moreira et al., Reference Moreira, Menegat, Barros, Bernardi, Wentz and Bortolozzo2017). However, even with the mobilisation of fat reserves before farrowing, sows with low feed intake produced less colostrum and litter weight gain in the first 24 h (Decaluwe et al., Reference Decaluwe, Maes, Cools, Wuyts, De Smet, Marescau, De Deyn and Janssens2014). Sows fed a lactation diet starting on day 104 of gestation produced more colostrum compared with sows fed a gestation diet (Garrison et al., Reference Garrison, van Heugten, Wiegert and Knauer2017). In contrast, no difference in piglet colostrum intake or sow colostrum yield was observed due to supplemental fat type (Theil et al., Reference Theil, Flummer, Hurley, Kristensen, Labouriau and Sorensen2014) or increased Lys and energy (Gourley at al., Reference Gourley, Swanson, Woodworth, DeRouchey, Tokach, Dritz, Goodband and Frederick2019).
Colostrum quality, as measured by immunoglobulin G concentration, has increased when feeding sows a tall oil fatty acid supplement (conjugated linoleic acid source) starting on day 107 of gestation (Hasan et al., Reference Hasan, Saha, Junnikkala, Orro, Peltoniemi and Oliviero2018) or high Lys and energy from day 113 of gestation to farrowing (Gourley et al., Reference Gourley, Swanson, Woodworth, DeRouchey, Tokach, Dritz, Goodband and Frederick2019). Colostrum immunoglobulin G was not increased with increased dietary fibre (Loisel et al., Reference Loisel, Farmer, Ramaekers and Quesnel2013). Thus, increased sow energy or amino acid intake in the few days prior to farrowing, during colsotrogenesis, can be beneficial to the colostrum quality.
Fibre use as an energy source in the peripartum transition period
Several studies have investigated the effects of dietary fibre during the transition period and its influence on colostrum yield, piglet survival and lactation performance. Loisel et al. (Reference Loisel, Farmer, Ramaekers and Quesnel2013) fed a low- (13.3% total dietary fibre) or high- (23.4% total dietary fibre) fibre diet to pigs from day 106 of gestation until parturition. They observed that low-birthweight pigs (<900 g) from sows fed high-fibre diets had increased colostrum intake, increased colostrum lipid concentrations and a reduction in pre-weaning mortality (14.7% v. 6.2%), but decreased colostrum immunoglobulin A concentrations, and no difference in total sow colostrum yield (3.9 v. 3.8 kg). Feyera et al. (Reference Feyera, Hojgaard, Vinther, Bruun and Theil2017) fed a dietary fibre-rich supplement (22% crude fibre) to pigs from day 102 of gestation to farrowing (280 g/day from day 102 to 108, and 570 g/day from day 109 to farrowing) and observed a reduction in stillbirths (8.8% v. 6.6%) and decreased piglet death from low viability (2.8% v. 1.5%) compared with sows fed a control diet (4.1% crude fibre). The researchers attributed the decrease in stillbirths to a greater amount of short-chain fatty acids available as energy in the colon, or from a reduction in sow constipation. Oliviero et al. Reference Oliviero, Kokkonen, Heinonen, Sankari and Peltoniemi(2009) demonstrated that increased fibre feeding pre-farrowing (7% v. 3.8% crude fibre) reduced constipation around parturition. Guillemet et al. Reference Guillemet, Guerin, Richard, Dourmad and Meunier-Salaun(2010) observed that sows fed a high-fibre diet in gestation (12.8% v. 3.5% crude fibre) transitioned more rapidly to a nutrient-dense lactation diet and tended to lose less backfat during the lactation period. However, fibre inclusion during the last 8 to 10 days before farrowing has not been shown to impact birthweight, litter gain, colostrum yield or metabolic criteria of the sow (Loisel et al., Reference Loisel, Farmer, Ramaekers and Quesnel2013; Feyera et al., Reference Feyera and Theil2017). Therefore, added fibre during transition may help transition a sow to a lactation diet and reduce stillbirths, but with limited to no impact on colostrum or litter growth.
Amino acids in the peripartum transition period
Fetal growth (22.7%), mammary growth (16.8%) and colostrum production (16.1%) represent the majority of the total required standardised ileal digestible (SID) Lys in late gestation, with the remaining requirement for oxidation/transamination, maintenance and uterine components (Feyera and Theil, Reference Feyera and Theil2017). These researchers predicted that relative to day 104 of gestation, the SID Lys requirement increased 149% by day 115 of gestation to approximately 35 g of SID Lys per day (Figure 3). This requirement is a significant increase compared with Lys typically provided in commercial production today. Therefore, the sow is likely in a negative Lys balance in the last few days before parturition. Mammary growth increases rapidly in the 10 days prior to farrowing, and will continue to increase up to day 10 of lactation (Kim et al., Reference Kim, Hurley, Han and Easter1999). The number of pigs determines the amount of Lys and amino acids required, and the sow will mobilise body fat and protein to support litter growth if her feed intake or diet quality is inadequate (Theil, Reference Theil and Farmer2015). Recently, it has been demonstrated that birth weight can be increased in gilts by supplying 40 g SID Lys per day beginning on day 107 or 113 of gestation (Gourley et al., Reference Gourley, Swanson, Woodworth, DeRouchey, Tokach, Dritz, Goodband and Frederick2019). Additionally, if fetal growth requirements are met, the female will partition increased nutrient intake towards backfat (Garrison et al., Reference Garrison, van Heugten, Wiegert and Knauer2017; Gourley et al., Reference Gourley, Swanson, Woodworth, DeRouchey, Tokach, Dritz, Goodband and Frederick2019). It is unknown from these studies whether body protein also increased during this period, but it is well understood that a gilt’s requirement for maternal body protein is greater compared with older parity females (Trottier et al., Reference Trottier, Johnson, de Lange and Farmer2014). Thus, gilts may benefit more from an increase in Lys and amino acids in the transition period due to partitioning towards body protein reserves and fetal growth. There is limited data during the transition period to understand the importance of amino acids besides Lys; however, Kim et al. (Reference Kim, Hurley, Wu and Ji2009) suggested that in late gestation, the sow requires increased amounts of arginine and leucine for fetal and mammary parenchymal tissues. Therefore, while high dietary Lys can be beneficial during the transition period, more research is needed to understand if additional amino acids will be of benefit for colostrum production and fetal growth.
Lactation
Although lactation represents only 15% to 20% of the productive cycle of a sow, it is undeniably the most metabolically demanding stage of production. The sow’s priority in lactation is to sustain milk production for the large and fast-growing litter of piglets, but is often not solely attained by voluntary feed intake. The mobilisation of body fat and protein reserves appears to be critical to support milk production in high-producing sows, although it is unclear whether body mobilisation is an obligatory process in modern sows (Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019). The typical negative effects of severe catabolism in lactation on the subsequent reproductive performance of sows is well established (Koketsu et al., Reference Koketsu, Dial, Pettigrew, Marsh and King1996), but modern sows seem to be more resilient to the effects of lactational catabolism (Patterson et al., Reference Patterson, Smit, Novak, Wellen and Foxcroft2011). This distinctive characteristic of the modern sow can be related to changes in biology and body lean composition, although sow resilience over successive parities has not been widely evaluated. Therefore, the main goal of the nutrition program for lactating sows should be to maximise feed intake to sustain milk production, without excessive mobilisation of BW reserves.
Energy requirements in lactation
The energy requirements of the modern lactating sow have increased significantly along with a marked increase in the number of piglets nursed. Milk production represents 65% to 80% of the energy requirements of lactating sows (Figure 4; National Research Council, 2012) and is the reason for an abrupt threefold increase in energy requirement within the first week of farrowing. The energy demand during lactation can impose a metabolic challenge to sows (Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019). If energy intake is insufficient, sows prioritise and sustain milk production at the expense of their own body reserves (Table 1). Energy intake is typically lower than lactation requirements, resulting in sows with a negative energy balance during most of lactation (Figure 4; NRC, 2012). This demonstrates the biological inability of lactating sows to consume enough feed to meet the energy requirements and, at the same time, presents an opportunity to develop nutritional strategies to stimulate sows to achieve an optimal level of energy consumption with minimal mobilisation of body reserves.
Table 1 Estimated daily milk production and mobilisation of body reserves1 of lactating sows according to the number of piglets nursed per sow and weight at weaning
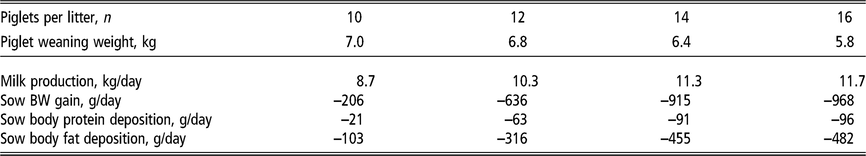
1 Estimates derived from the NRC (2012) model assuming a feeding level of 6.5 kg/day of a lactation diet containing 13.8 MJ metabolisable energy per kilogram in a 21-day lactation for multiparous sows. Piglet growth rate estimated from published studies prior to the genetic selection for piglet birth weight (Beaulieu et al., Reference Beaulieu, Aalhus, Williams and Patience2010; Huber et al., Reference Huber, de Lange, Krogh, Chamberlin and Trottier2015; Fan et al., Reference Fan, Yang, Kim, Menon and Baidoo2016; Strathe et al., Reference Strathe, Bruun and Hansen2017a; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019), which is expected to increase piglet weaning weight.
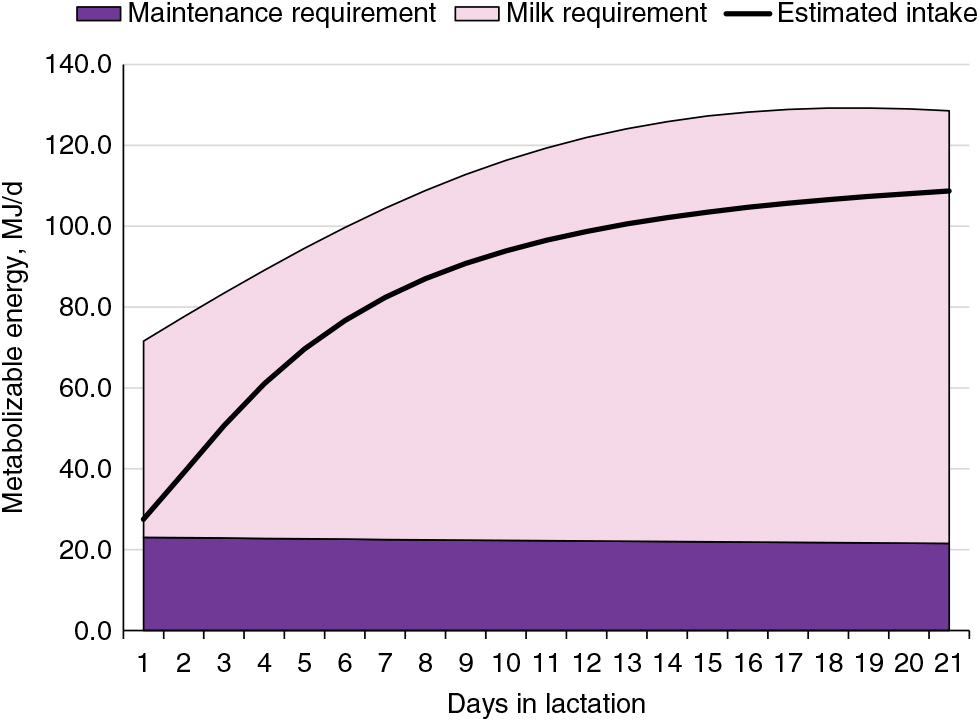
Figure 4 Energy requirement estimates for maintenance and milk production and estimated energy intake of lactating sows. Estimates were derived from the NRC (2012) assuming 14 piglets per litter and 6.4 kg piglet weaning weight in a 21-day lactation for multiparous sows.
The energy concentration of lactation diets is an important determinant of energy consumption and is typically modified by the use of fats, oils or fibres in the diet. An increase in dietary energy concentration typically represents an increase in energy intake at the same feed intake until a level at which the dietary energy concentration negatively affects feed intake (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012). Studies demonstrated that increasing the energy concentration of lactation diets from 12.8 to 13.4 MJ ME/kg improved energy intake and consequently reduced weight loss and increased litter growth rate during lactation (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012). However, lactation diets with a high energy concentration of 13.8 to 14.2 MJ ME/kg had a negative impact on feed intake (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012) and, thus, did not further increase energy intake.
Increasing energy density with fats or oils is a nutritional strategy that seems to be particularly important for lactating sows under heat stress conditions (Rosero et al., Reference Rosero, van Heugten, Odle, Arellano and Boyd2012) and for prolific and high-producing lactating sows (Strathe et al., Reference Strathe, Bruun and Hansen2017a). In a literature review, the addition of 2% to 11% fats and oils in lactation diets improved the energy intake of sows by an average of 7% or 4.6 MJ ME per day (Rosero et al., Reference Rosero, Boyd, Odle and van Heugten2016). As sows prioritise lactation needs, the additional energy is preferentially partitioned for milk and converted as milk fat output (Rosero et al., Reference Rosero, Odle, Mendoza, Boyd, Fellner and van Heugten2015). Consequently, the benefits of greater energy intake are observed as improvements in litter growth rate because of a greater amount of energy provided through the milk (Rosero et al., Reference Rosero, Odle, Mendoza, Boyd, Fellner and van Heugten2015, Reference Rosero, Boyd, Odle and van Heugten2016). Similarly, lactation diets with high levels of dietary fibre resulted in a reduction in energy intake (Schoenherr et al., Reference Schoenherr, Stahly and Cromwell1989). Fibrous diets have low energy and bulk density, which physically restrict a sow’s ability to consume the volume of feed necessary to achieve a high energy intake (Schoenherr et al., Reference Schoenherr, Stahly and Cromwell1989).
In summary, the addition of high-energy ingredients to lactation diets allows an increase in energy intake and energy output in milk. Consequently, there is a reduction in BW loss and an improvement in litter growth rate during lactation.
Amino acid and protein requirements in lactation
The amino acid requirements of high-producing lactating sows have increased substantially to support the milk production demand of large litters. The number of piglets nursed per sow as well as the litter growth rate during lactation dictate the amino acid requirements of lactating sows (Table 2). The amino acids for milk production represent most of the requirements, as lactating sows utilise as much as 70% of dietary protein for milk protein synthesis (Pedersen et al., Reference Pedersen, Bruun, Feyera, Larsen and Theil2016). It appears that milk production is hardly changed by lactation diet because sows are able to mobilise body reserves (Noblet and Etienne, Reference Noblet and Etienne1987). However, the supply of dietary amino acids and CP close to the requirements can improve milk protein output (Strathe et al., Reference Strathe, Bruun, Geertsen, Zerrahn and Hansen2017b) and reduce muscle protein mobilisation in lactating sows (Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019). Recent studies underline that a dietary intake of both balanced protein and essential amino acids is mutually important to sow and litter performance during lactation (Strathe et al., Reference Strathe, Bruun and Hansen2017b; Huber et al., Reference Huber, Rudar, Trottier, Cant and de Lange2018; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019).
Table 2 Daily lysine requirement estimates1 (grams of standardised ileal digestible lysine per day) of lactating sows according to the number of piglets nursed per sow and weight at weaning
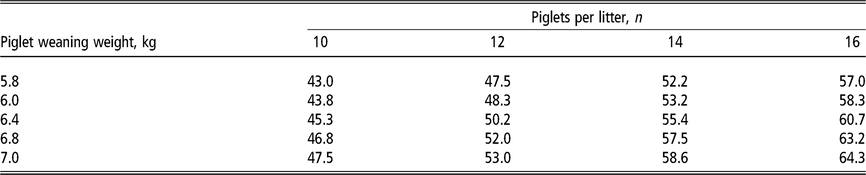
1 Estimates derived from the NRC (2012) model assuming a feeding level of 6.5 kg/day of a lactation diet containing 13.8 MJ metabolisable energy per kilogram in a 21-day lactation for multiparous sows. For primiparous sows, the lysine requirements in grams per day are approximately 5% lower due to lower milk production but approximately 5% higher as a diet percentage due to lower feed intake. Piglet growth rate estimated from published studies prior to the genetic selection for piglet birth weight (Beaulieu et al., Reference Beaulieu, Aalhus, Williams and Patience2010; Huber et al., Reference Huber, de Lange, Krogh, Chamberlin and Trottier2015; Fan et al., Reference Fan, Yang, Kim, Menon and Baidoo2016; Strathe et al., Reference Strathe, Bruun and Hansen2017a; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019), which is expected to increase piglet weaning weight.
Dietary intake of balanced protein supplies essential amino acids and nitrogen necessary to synthesise non-essential amino acids. The high-producing sow seems to benefit from a balanced protein intake during lactation by improving litter growth rate and reducing BW loss (Strathe et al., Reference Strathe, Bruun and Hansen2017b, Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019). Studies with high feed-grade amino acids suggested that increasing digestible CP up to 13.5% (approximately 15.5% CP) improved litter growth rate by increasing sow milk protein output (Strathe et al., Reference Strathe, Bruun and Hansen2017b). Higher levels of digestible CP of 14.3% (approximately 16.5% CP) seemed to minimise sow BW loss by sparing muscle protein mobilisation for the purpose of milk production (Strathe et al., Reference Strathe, Bruun and Hansen2017b). Thus, lactation diets may need a minimum digestible CP content of 13.5% to 14.3%.
Recently, several studies have evaluated amino acid requirements to ensure optimum performance of high-producing lactating sows. In general, the amino acid requirement estimates vary depending on performance criteria and statistical methodology applied in the study. Lysine requirement estimates are the most frequently studied, as models predict a substantial increase in Lys requirements of lactating sows with large, fast-growing litters (Table 2). The literature seems to agree on the effect of increasing dietary Lys intake to reduce BW loss and body protein mobilisation, but is conflicting in terms of the influence of dietary Lys intake on litter growth rate and subsequent reproductive performance (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). Studies using a range of 0.50 to 0.81 g SID Lys per MJ ME determined that the Lys requirement estimate to minimise sow BW loss in the lactation period is around 0.72 to 0.79 g SID Lys per MJ ME (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). Although the estimates seemed to be within the same range for primiparous and multiparous sows, the BW loss has been reported to be considerably greater in primiparous than multiparous sows, at around 12% (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015) and 7% (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017), respectively. The reduction in sow BW loss is presumably the consequence of a low mobilisation of muscle protein, as evidenced by a reduction in loin eye depth loss during lactation (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). Lower concentrations of plasma urea nitrogen and plasma creatinine as a result of increased Lys intake support a reduction in sow body protein utilisation and muscle catabolism (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012). However, there is no consensus on the effect of dietary Lys on body fat stores (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). It is proposed that the mobilisation of energy and protein are not completely independent. Thus, the interaction between amino acid and energy requirements is more complex and subject to factors involved in nutrient deficit, including energy and protein intake, energy and protein output in milk, growth rate of the litter and lactation length (Dourmad et al., Reference Dourmad, Étienne, Valancogne, Dubois, van Milgen and Noblet2008).
Milk production and milk composition are arguably the most important factors capable of stimulating and supporting an improvement in litter growth rate (Strathe et al., Reference Strathe, Bruun and Hansen2017b). However, the influence of dietary Lys intake on milk production and composition is not well understood. In a study with primiparous sows, milk protein content increased with dietary Lys levels up to 0.81 g SID Lys per MJ ME in a range of 0.55 to 0.81 g SID Lys per MJ ME (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015), but no other recent Lys requirement studies have evaluated sow milk composition (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). In contrast, an increase in milk protein content is not reflected in an improved growth rate of primiparous litters (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015). While some studies observed no influence of dietary Lys intake on the growth rate of primiparous litters (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017), others suggested an improvement in litter growth rate up to 0.72 to 0.79 g SID Lys per MJ ME for primiparous and multiparous sows (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). Estimating Lys requirements for litter growth rate is seemingly complex due to the capacity of sows to maintain milk production and sustain litter growth rate by mobilising body reserves (Noblet and Etienne, Reference Noblet and Etienne1987). Moreover, the estimation of Lys requirements for litter growth rate probably requires a multifactorial approach by taking into account parity, lactation curve, daily Lys intake, growth rate of the litter, milk production and milk composition, as these factors affect how Lys is required and partitioned by lactating sows.
Interestingly, the amount of daily digestible Lys intake per kilogram of litter daily gain is consistent around 24 to 25 g for the recent studies on Lys requirements to improve litter growth rate for lactating sows (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). Previous reviews conducted by Pettigrew (Reference Pettigrew1993) and Boyd et al. (Reference Boyd, Touchette, Castro, Johnston, Lee and Han2000) determined a positive correlation between increased Lys requirements and litter growth rate. The regression using published data from 1972 to 1997 indicated that 26 g of total Lys or approximately 22 g of digestible lysine intake per day is needed for each 1 kg of litter growth, and sows are expected to mobilise 8 g of Lys per day from body protein reserves (Boyd et al., Reference Boyd, Touchette, Castro, Johnston, Lee and Han2000). The original equation has been updated (Figure 5) with Lys requirements for optimal litter growth rate from published studies conducted between 1998 and 2017 with primiparous and multiparous sows (Sauber et al., Reference Sauber, Stahly, Williams and Ewan1998; Yang et al., Reference Yang, Pettigrew, Johnston, Shurson and Walker2000, Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). The new regression predicted an increase to 27 g per day in the amount of digestible Lys intake required for each 1 kg of litter growth, and also an increase to 13 g per day in the expected mobilisation of Lys from body protein reserves. This predicted increase in the estimates of both Lys requirement and mobilisation of reserves coincides with the expectation for modern sows, which are leaner and higher milk producers than sow genotypes in the past.
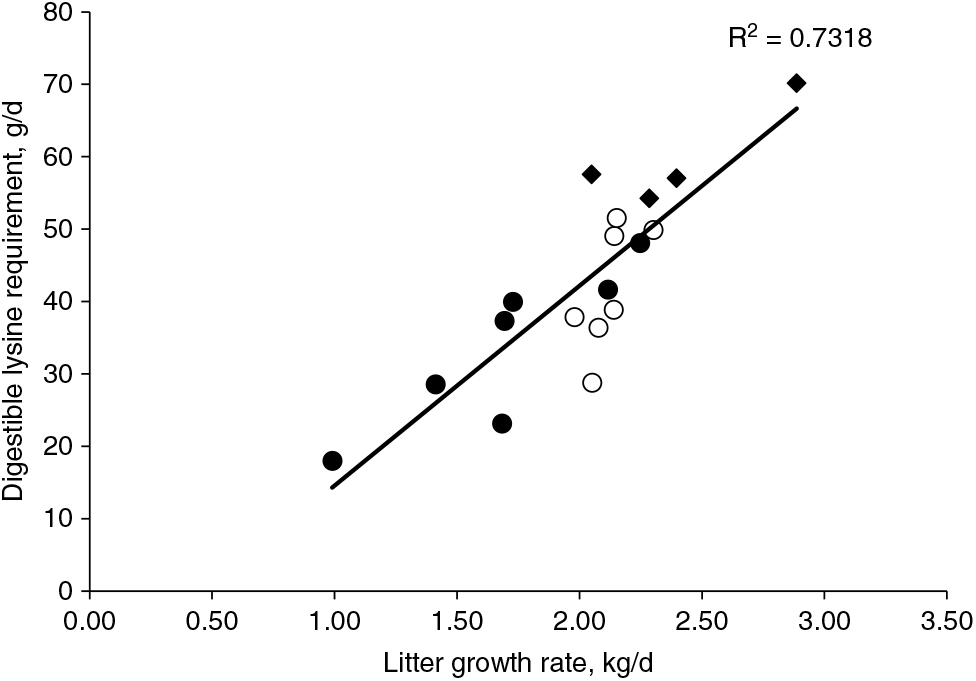
Figure 5 Regression curve to estimate the digestible lysine requirement to optimise litter growth rate from published studies. The regression curve originally derived from published lysine requirement studies from 1972 to 1997 summarised by Pettigrew (Reference Pettigrew1993) in the solid circles and Boyd et al. (Reference Boyd, Touchette, Castro, Johnston, Lee and Han2000) in the open circles. The present updated curve contains data from studies published from 1998 to 2017, represented by the diamonds. The updated regression indicates that 27 g of digestible lysine intake per day is needed for each 1 kg of litter growth, and sows are expected to mobilise 13 g of lysine per day from body protein reserves.
It is well recognised that excessive weight loss and mobilisation of body reserves during lactation are associated with a prolonged wean-to-oestrus interval and inferior subsequent reproductive performance in sows (King, Reference King1987; Koketsu et al., Reference Koketsu, Dial, Pettigrew, Marsh and King1996). Thus, the attenuation of lactational catabolism with an increase in dietary Lys intake in lactating sows (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017) has been intuitively related to improvements in subsequent reproduction. Early studies consistently demonstrated the effect of amino acid intake on improving wean-to-oestrus interval and litter size (King, Reference King1987; Touchette et al., Reference Touchette, Allee, Newcomb and Boyd1998), mediated by the release of reproductive and metabolic hormones (King and Martin, Reference King and Martin1989; Tokach and Dial, Reference Tokach and Dial1992). However, the influence of dietary Lys intake on subsequent reproductive performance of modern sows is not as clear based on recent studies. There is evidence to suggest an improvement in the secretion of estradiol and luteinising hormone in primiparous and multiparous sows around the peak of lactation with dietary Lys levels of 0.72 to 0.79 g SID Lys per MJ ME (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012). These hormones play an important role in follicular development during lactation and cyclicity return after weaning (Soede et al., Reference Soede, Langendijk and Kemp2011). Indeed, the same study demonstrated a short wean-to-oestrus interval with dietary Lys levels of 0.72 to 0.79 g SID Lys per MJ ME (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012). However, there is no consensus in the literature (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). For primiparous sows, Gourley et al. (Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017) fed dietary SID Lys of 0.52 to 0.81 g per MJ ME and observed an improvement in the number bred within 7 days after weaning; however, the effect on wean-to-oestrus interval is not consistent (Xue et al., Reference Xue, Piao, Li, Li, Zhang, Kim and Dong2012; Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015; Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017). The effect of dietary Lys on reproductive hormones during the first lactation was not evident in another recent study (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015). Likewise, dietary Lys levels during lactation did not seem to have an influence on the conception rate (Shi et al., Reference Shi, Zang, Li, Shi, Liu, Zhu and Li2015) or the number of piglets born in the subsequent parturition (Gourley et al., Reference Gourley, Nichols, Sonderman, Spencer, Woodworth, DeRouchey, Dritz, Goodband, Kitt and Stephenson2017).
The lack of a clear influence of dietary Lys intake during lactation on reproduction in the subsequent cycle seemed to corroborate with the remark that the reproductive performance of modern primiparous sows is increasingly resilient to the negative effects of tissue catabolism during lactation (Patterson et al., Reference Patterson, Smit, Novak, Wellen and Foxcroft2011). Greater protein reserves of modern sows may provide more reserves to limit the dietary amino acid influence on subsequent reproduction.
The requirements of essential amino acids in milk and mammary gland tissue increase as the number of piglets nursed increases (Kim et al., Reference Kim, Baker and Easter2001). The most limiting amino acids for milk production are typically Lys, threonine and valine (Kim et al., Reference Kim, Baker and Easter2001; Soltwedel et al., Reference Soltwedel, Easter and Pettigrew2006); thus, the requirements of the latter amino acids as a ratio to Lys have been recently re-evaluated for high-producing lactating sows. The threonine requirement estimate to optimise the litter growth rate of lactating sows was approximately 65% of SID Lys with a range of 52% to 84% (Greiner et al., Reference Greiner, Srichana, Usry, Neill, Allee, Connor, Touchette and Knight2018). However, the lack of other threonine requirement studies with modern lactating sows hinders the validation of threonine requirement estimates.
Recent studies did not reach a consensus about the requirement estimates of valine as a ratio to Lys. Valine concentrations above 76% of SID Lys provide no improvement in litter growth rate and sow backfat loss in a valine range of 76% to 97% of SID Lys (Strathe et al., Reference Strathe, Bruun, Zerrahn, Tauson and Hansen2016). However, an improvement in both criteria was evident with very high levels of valine (113% of SID Lys) for litter growth rate and 88% of SID Lys for minimising backfat loss (Xu et al., Reference Xu, Zeng, Xu, Tian, Ma, Long, Piao, Cheng and Piao2017). The requirement for valine in lactating sow diets seemed to be independent of total branched-chain amino acid concentrations, indicating that leucine and isoleucine do not spare the requirement of valine for sows in lactation (Moser et al., Reference Moser, Tokach, Dritz, Goodband, Nelssen and Loughmiller2000).
The requirement for tryptophan for lactating sows has been estimated to be 22% of SID Lys to maximise feed intake and at 26% of SID Lys to minimise BW loss in primiparous sows, with no effect on multiparous sows (Fan et al., Reference Fan, Yang, Kim, Menon and Baidoo2016). However, similar to threonine, the lack of other tryptophan requirement studies with modern lactating sows hinders the validation of tryptophan requirement estimates. Furthermore, studies evaluating the requirements of branched-chain amino acids and sulphur-containing amino acids, among others, for high-producing lactating sows are non-existent in recent literature.
The variation in amino acid requirements for lactating sows could be a consequence of the dynamic body tissue mobilisation during lactation (Kim et al., Reference Kim, Hurley, Wu and Ji2009). The ideal dietary amino acid profile for lactating sows is influenced by the amino acid profile in milk and mammary gland tissue, and the amino acid resulting from body tissue mobilisation (Kim et al., Reference Kim, Baker and Easter2001). Because of these differences, threonine is a critical amino acid for sows with low lactation feed intake and substantial mobilisation of body reserves during lactation, whereas valine is an important amino acid for sows with high feed intake and limited mobilisation of body reserves during lactation (Kim et al., Reference Kim, Baker and Easter2001; Soltwedel et al., Reference Soltwedel, Easter and Pettigrew2006). Although the second- and third-limiting amino acids for lactating sows vary according to body tissue mobilisation, Lys is consistently the first-limiting amino acid (Kim et al., Reference Kim, Baker and Easter2001; Soltwedel et al., Reference Soltwedel, Easter and Pettigrew2006).
In summary, the dietary provision of amino acids close to the requirements of lactating sows allows a reduction in body protein mobilisation and has the potential to improve litter growth rate. The influence of amino acid intake on sow and litter performance seems to be even more complex for primiparous sows, as recent studies failed to report an amino acid-derived improvement in performance during the first lactation.
Calcium and phosphorus requirements in lactation
Calcium and phosphorus requirements for high-producing lactating sows have been currently estimated using a modelling approach (NRC, 2012). A scarcity of recent research prevents the validation of model-derived requirement estimates. The dynamic mobilisation of calcium and phosphorus in catabolic sows during lactation adds complexity to their requirement estimates using empirical studies.
The requirement estimates of calcium and phosphorus for lactating sows are primarily influenced by milk production (NRC, 2012). High-producing lactating sows with large, fast-growing litters have a considerable increase in calcium and phosphorus requirements (Table 3) in order to support their demand in milk production (Table 4). Moreover, calcium and phosphorus requirements are expected to increase throughout the lactation period following the sow milk production curve. The dietary intake of calcium and phosphorus is of great importance for primiparous sows to support their growth and development of bone and muscle tissues (NRC, 2012). Moreover, calcium and phosphorus are likely more critical for primiparous sows that might not have these mineral reserves for mobilisation as a multiparous sow.
Table 3 Daily phosphorus requirement estimates1 (grams of standardised total tract digestible phosphorus per day) of lactating sows according to the number of piglets nursed per sow and weight at weaning
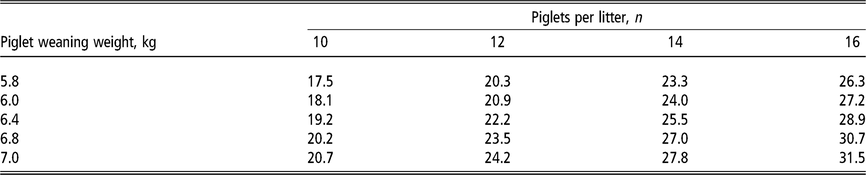
1 Estimates derived from the NRC (2012) model assuming a feeding level of 6.5 kg/day of a lactation diet containing 13.8 MJ metabolisable energy per kilogram in a 21-day lactation for multiparous sows. For primiparous sows, phosphorus requirements in grams per day are approximately 5% lower due to lower milk production but approximately 5% higher as a diet percentage due to lower feed intake. Total calcium intake is estimated at two times the digestible phosphorus requirement. Piglet growth rate estimated from published studies prior to the genetic selection for piglet birth weight (Beaulieu et al.,Reference Beaulieu, Aalhus, Williams and Patience2010; Huber et al., Reference Huber, de Lange, Krogh, Chamberlin and Trottier2015; Fan et al., Reference Fan, Yang, Kim, Menon and Baidoo2016; Strathe et al., Reference Strathe, Bruun and Hansen2017a; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019), which is expected to increase piglet weaning weight.
Table 4 Estimated daily calcium and phosphorus output1 in sow milk according to the number of piglets nursed per sow and weight at weaning
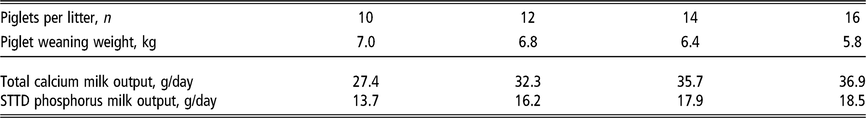
STTD = standardised total tract digestible.
1 Estimates derived from the NRC (2012). Milk phosphorus is predicted from milk nitrogen output at a ratio between standardised total tract digestible phosphorus and nitrogen of 0.196. Milk calcium is predicted from milk phosphorus output at a ratio between total calcium and standardised total tract digestible phosphorus of 2. Piglet growth rate estimated from published studies prior to the genetic selection for piglet birth weight (Beaulieu et al., Reference Beaulieu, Aalhus, Williams and Patience2010; Huber et al., Reference Huber, de Lange, Krogh, Chamberlin and Trottier2015; Fan et al., Reference Fan, Yang, Kim, Menon and Baidoo2016; Strathe et al., Reference Strathe, Bruun and Hansen2017a; Pedersen et al., Reference Pedersen, Chang, Trottier, Bruun and Theil2019), which is expected to increase piglet weaning weight.
Practical considerations in feeding programs
Diet formulation is only one step in developing a feeding program for today’s sow. High feed intake is necessary to meet the energy and amino acid requirements of high-producing sows. The feeding system, environment, sow body condition and choice of ingredients will influence daily feed intake during lactation and have as much impact on sow productivity as nutrient levels in the diet.
Advances in feed delivery systems
Producers and researchers have long debated whether feed should be gradually increased during the first week of lactation or provided ad libitum immediately after farrowing. Research in this area is not new, but continually showed that ad libitum feeding mostly results in a higher feed intake over the entire lactation phase than step-up programs (Stahly et al., Reference Stahly, Cromwell and Simpson1979; Moser et al., Reference Moser, Cornelius, Pettigrew, Hanke, Heeg and Miller1987). The increased size of swine facilities coupled with advances in equipment design have made ad libitum feed delivery a reality in most large production systems.
Environment and sow intake
Sows maintained in the thermoneutral zone will have a higher feed intake than sows experiencing heat stress. McGlone et al. Reference McGlone, Stansbury and Tribble(1988) demonstrated that drip coolers were more effective at relieving heat stress than snout coolers or increases in diet energy density. Black et al. Reference Black, Mullan, Lorschy and Giles(1993) summarised that ‘increasing heat loss from the sow, particularly through increasing the area of wet skin, has a greater positive effect on animal performance than modifying the diet’. An increased use of evaporative cool cells and drip coolers allows farms in hot climates to greatly increase feed intake compared to not using these technologies.
Gestation body condition
Numerous studies have demonstrated that sows with a higher backfat at farrowing have a lower feed intake during lactation than sows with a lower backfat at farrowing. Dourmad (Reference Dourmad1993) found that providing high levels of feed intake during gestation decreased lactation feed intake by resulting in smaller meals and shorter feeding duration. Increasing the fibre in gestation diet, while providing the same energy intake, increased meal frequency during lactation, but did not increase feed intake (Guillemet et al., Reference Guillemet, Dourmad and Meunier-Salaun2006). Data from more modern sows (Kim et al., Reference Kim, Yang, Pangeni and Baidoo2015) illustrate that lactation feed intake decreases linearly as backfat before farrowing increases, with the greatest decrease in feed intake for sows with >20 mm of backfat at farrowing.
Producers understand the importance of maintaining sows in the correct body condition, but have difficulty achieving the goal in the field. Sows are often over- or underconditioned on individual farms. Although ultrasound is a better tool to assess sow backfat than body condition score (Young et al., Reference Young, Tokach, Aherne, Main, Dritz, Goodband and Nelssen2004), it can be too time-consuming and difficult to accomplish in the field. The invention of a sow caliper (Knauer and Baitinger, Reference Knauer and Baitinger2015) provides a fast, unbiased tool for producers to assess body condition.
Phase feeding
The information provided in this review suggests that phase feeding may provide benefits for lactating sows. A peripartum diet fed prior to and immediately after farrowing may be targeted towards reducing stillbirths and encouraging sow feed consumption. A lactation diet, fed for the remainder of lactation, would be designed for optimal milk production and subsequent reproduction. The use of a lower nutrient-dense diet until day 10 after farrowing lowered feed cost, but did not influence the performance of sows in a Danish commercial study (Sorensen, Reference Sorensen2007). Similarly, Craig et al. Reference Craig, Henry and Magowan(2016) found that feeding a constant energy level during lactation resulted in similar performance to sows that were offered a lower energy diet before day 14 and a higher energy diet after day 14 of lactation. Conversely, Pedersen et al. Reference Pedersen, Bruun, Feyera, Larsen and Theil(2016) found that altering the diet to meet the sows’ changing requirements as lactation progressed increased sow milk yield and pig weaning weight compared with feeding a single lactation diet; however, the single lactation diet used in the study was below the sow’s requirement for amino acids for much of lactation. Thus, more research is needed to determine if providing two different diets during lactation provides any productivity benefits compared with feeding a single lactation diet that more closely meets the sows’ requirements.
Conclusion
In summary, the lactating sow has demonstrated remarkable resiliency in the face of rapid improvements in production and nutritional challenges. Many practical aspects of meeting the nutrient requirements of high-producing sows have not changed. With increased milk production, amino acid and energy requirements must be met in order to avoid excessive body tissue catabolism. Future research needs to continue to improve our understanding of sow’s requirements during the peripartum transition period to reduce farrowing duration and increase pig survival. Our knowledge of these and other facets of sow management will ultimately improve the welfare of the sow and her offspring.
Acknowledgements
The authors thank Dr. Matt Culbertson at Genus PIC for providing genetic trend data, and Elsevier for allowing the use of copyrighted material.
Michael D Tokach, 0000-0002-9621-362X
Declaration of interest
There are no conflicts of interest to declare.
Ethics committee
All work is within guidelines and requirements of ethics committees.
Software and data repository resources
None of the data were deposited in an official repository.













