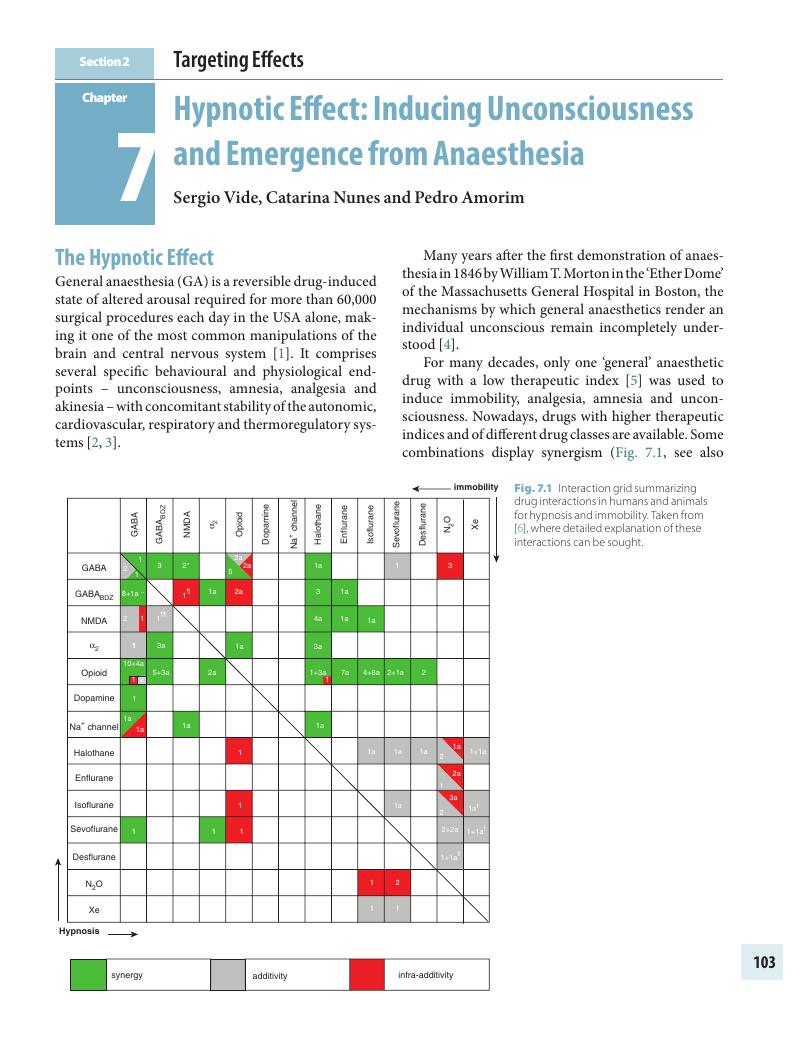
Section 2 - Targeting Effects
Published online by Cambridge University Press: 03 December 2019
Summary
- Type
- Chapter
- Information
- Personalized AnaesthesiaTargeting Physiological Systems for Optimal Effect, pp. 103 - 290Publisher: Cambridge University PressPrint publication year: 2020



