7.1 Can Plants Tell Stories?
This is the question Darwin set out to answer in his final book, The Power of Movement in Plants (Reference Darwin and DarwinDarwin and Darwin 1880), published shortly before he died. This idiosyncratic question summed up Darwin’s life-long attempt to understand the common history of all life, and to devise strategies for telling it. Using a variety of innovative techniques, Darwin eventually figured out how to record what he termed ‘the life-history of the plant’, putting special emphasis on the way plants interacted with the world around them, sensing changes in environment, reacting to stimuli, deciding their fate (Reference Darwin and DarwinDarwin and Darwin 1880: 548). Darwin’s argument for the ability of plants to feel and react, even to think, was controversial in his time, but opened up entirely new avenues of research into plant physiology, from plant signalling (the relay of information), to chemotaxis and tropism (respectively, movement and growth in response to stimuli). Today, research into these phenomena is commonplace. But I wish to take up perhaps the most radical implication of Darwin’s plant studies: plants do have stories to tell, and if we listen closely, they can tell them to us.
These stories bear little comparison to a Jane Austen novel; in the stories Darwin recorded, we do not find plant-based Elizabeth Bennets, waiting to see whether Mr. Darcy will deign to join the dance. But these narratives do catch a perhaps more delicate interaction in which, as literary historian Gillian Beer has put it, ‘observer and observed are in a dance of accord’ (Reference Beer and FaflakBeer 2017: 31). Drawing on the history and philosophy of science, performance studies and narrative theory, I will explore the implications of Darwin’s plant studies for the place of narrative in science.
It is generally recognized that Darwin’s scientific accounts were organized by narratives – various stories that attempted to explain how specific relationships, structures and behaviours evolved in the past (Reference LevineLevine 1988; Reference BeerBeer 2000). The key role that narratives play in Darwin’s accounts underlines the importance of narratives to science in general, but also the importance of considering how scientific narratives are structured by wider practices of storytelling. In my earlier studies of Darwin’s science, I have emphasized the necessarily fictive quality of the stories produced by Darwin’s studies, insofar as they retroject a persuasive narrative on the basis of incomplete evidence (Reference GriffithsGriffiths 2016). As Greg Priest points out, these ‘conjectural historical narratives’ were sometimes organized by Darwin into diagrams, as in the famous tree of life from the Origin (Reference PriestPriest 2018). Darwin described the stories he imagined as ‘castles in the air’, retrospective fictions tethered to empirical grounds through the meticulous but necessarily partial assembly of historical data and present observations. In this way, we might take Darwin’s castles as proof of the claim that new scientific narratives, and new scientific theories in general, are produced by the scientific imagination; they are, as Alistair Crombie put this, ‘designed in the mind’ (Reference CrombieCrombie 1988). Similarly, Erin James has suggested that narratives about plants tend to stage a kind of ventriloquist act, in which plants serve as a vehicle for the expression of human opinions and perspectives (Reference James, Gagliano, Ryan and VieiraJames 2017).
The present chapter departs from this line of human-centred thinking, by asking: to what degree were Darwin’s narratives recordings of narratives from nature itself? And how did the objects of Darwin’s studies intervene in telling their own stories? Darwin’s narratives often operate on at least two levels. On the one hand, he hypothesized long-term stories of adaptive evolution to bridge the evidentiary gaps in the distribution of traits within current and past species, explaining how complex behaviours and traits might evolve from simpler precursors. But in his later works he also placed increasingly heavy emphasis on the contingent and idiosyncratic way that specific traits were adapted to new purposes. And he sought to document this contingency, which operated at the level of species history, through smaller-scale narratives that described the lives of individual specimens, in detailed micro-histories of their growth and contingent change. Darwin’s last major work, The Power of Movement in Plants, marks the culmination of these efforts. In allowing plants to draw their contingent behaviour on the page, he enlisted them in his efforts to narrate (from the perspective of individual plants) how they grow, subsequently articulating these accounts to reconstruct (from the perspective of the species) how they once evolved.
Central to that approach was a set of techniques that allowed plants to inscribe their growth on various media, writing their lives into Darwin’s science. Figure 7.1 is a late example – a graphic reproduction of the trail a plant root left on a glass slide as it grew. What would it mean to read these graphic traces as scientific narratives? Narrative theorist Mieke Bal defines narrative as ‘a text in which an agent relates (“tells”) a story in a particular medium’. Bal further defines ‘story’ as a set of ‘chronologically related events that are caused or experienced by actors’, emphasizing that agents are ‘not necessarily human’ and that the ‘medium’ may be visual as much as textual (Reference BalBal 1997: 4). Darwin’s plant illustrations certainly seem to meet a minimal description of narrative as a linear account of events experienced by some agent. Here, we see in the various squiggles, and in the alteration of thicker and thinner strokes, the varying pressure and direction of the root of a plant as it senses and attempts to grow around the slide. And yet, to take these illustrations seriously as narratives, as pieces of the ‘life-history of the plant’, we must rethink our basic intuitions about where scientific narratives come from. More than the narratives about science that Robert Meunier discusses (Chapter 12), or the ‘narratives of nature’ that John Huss studies (Chapter 3), they are narratives from nature. Such scientific narratives are not simply produced by the scientists and projected on the world, but rather are generated through interaction with that world, elaborated through what Andrew Pickering has termed a ‘dance of agencies’ – both natural and human (Reference PickeringPickering 1995).
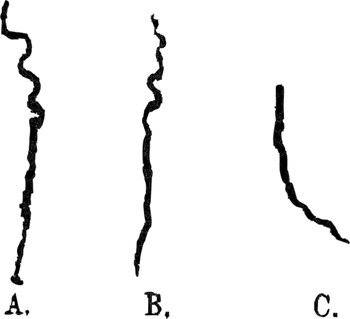
Figure 7.1 Phaseolus multiflorus
‘Tracks left on inclined smoked glass-plates by tips of radicles in growing downwards. A and C, plates inclined at 60°, B inclined at 68° with the horizon’.
The following chapter has two movements. In the first, I’ll explore the methods of Darwin’s plant science, attending to the performative intricacy of scientific experimentation as a collaborative dance that highlights the contingent, narrative aspects of plant development – akin to the ‘reticulate approach’ identified by Elizabeth Haines (Chapter 9). In the section that follows, I’ll examine how these contingent histories, co-elaborated by Darwin and his plant subjects, are articulated together at the close of the work as a ‘life-history’ – in effect, a Bildungsroman that reconfigures individual events in relation to an overarching evolutionary thesis. On the basis of this account, I propose a way of understanding scientific narratives as moving between entanglement with the world and reconceptualization, and between the narration of contingent events and their reconfiguration into higher-order narrative genres, by means of a process that draws multiple actors, human and non-human, into alignment, cultivating multi-level, multiply-scaled stories about how the world works.
7.2 Plant Narrative and the ‘Dance of Agencies’
At the core of Darwin’s botanical research program was the question of how plants and other forms of life interact. Over the course of several decades, especially after his move to Down House, some fifteen miles from London, Darwin let his imagination run riot, exploring an astonishing range of methods to entice plants into cooperating with his studies. He tickled them with horse hair and pencil; he gave them a spin in rotating boxes; he played them music; he fed them sweetmeats, and sometimes, just meat. These experiments, simple, elegant, intimate, produced results that often astonished the botanical world, and the greenhouse at Down became an object of fascination for visitors. The results were published in a series of pathbreaking works of botany (Reference DarwinDarwin 1862b; Reference 162Darwin1865; Reference Darwin1875a; Reference Darwin1875b; Reference Darwin1876; Reference Darwin1877; Reference Darwin and DarwinDarwin and Darwin 1880).
All of Darwin’s plant studies demonstrate a fascination with the relation between plant and animal life, and Darwin’s insistent assertions that plants possessed sensitivity, an ability to move, digest and even think, much like animals. Darwin’s grandfather, Erasmus Darwin, was famous for drawing analogies between plant and animal life, between their modes of reproduction and growth, and was eventually notorious for insisting that these analogies indicated a common nature and a more basic, shared history of evolution (Reference PriestmanPriestman 2000). In adopting his grandfather’s commitment to the shared nature of plant and animal life, Charles took an unusual perspective on the possibilities of plant agency, and a unique interest in documenting their sensitive and responsive engagement with the world – sensing and capturing insects for nourishment, grasping and then climbing neighbouring trees and structures.
As Darwin exposed increasingly complex plant behaviours and adaptations, others argued that these elaborate behaviours defied explanation by the gradual means of natural selection. In 1871, St. George Jackson Mivart summed up these objections, arguing that, even if natural selection might operate on such adaptations after they evolved, it could not explain how they first developed. The elaborate adaptive structures of orchids, and the power of twining plants to climb trees, illustrated the ‘incompetency of “natural selection” to account for the incipient stages of useful structures’ (Reference MivartMivart 1871: 35).Footnote 1 It was the most succinct statement of what Stephen Jay Gould would later term the ‘5 percent of a wing principle’: variations in the wing structure of flying birds might experience selective pressure, but an incipient wing would seem to be useless for flight and therefore non-adaptive (Reference GouldGould 2002: 1220).
Darwin recognized this as a serious challenge to the comprehensiveness of the theory of natural selection and immediately set out to answer it. The following year, he added an entirely new chapter to the sixth edition of On the Origin of Species, responding at length to Mivart’s critiques. In the only new chapter ever added to that work, Darwin placed heavy emphasis on the sensitive actions of plants as examples in which the ‘incipient stages of useful structures’ might have developed ‘incidentally’ from other adaptive traits (Reference DarwinDarwin 1872: 198). All plants, he noted, seemed to have some capacity to move, and this movement is often coordinated with a basic sensitivity to specific influences, like sunlight and gravity. This innate sensitivity gave them an ‘incidental’ sensitivity to touch, much as ‘the nerves and muscles of an animal are excited by galvanism’ or electrical stimulus, despite such sensitivity being non-adaptive (Reference DarwinDarwin 1872: 198). These incidental abilities, Darwin argued, could be the building blocks of much more complex adaptive behaviours, like the behaviour of climbing and insect-eating plants.
As Gould explains, this marked a significant shift in the emphasis Darwin placed on such ‘exaptations’ – a term coined by Gould and Elisabeth S. Vrba to describe cases of functional repurposing (Reference Gould and VrbaGould and Vrba 1982). As Gould later explained, Darwin gave exaptation a ‘vital role in establishing the contingency and unpredictability of evolutionary change’, with the consequence that ‘historical [i.e., narrative] explanation’ became central to his evolutionary histories (Reference GouldGould 2002: 1224–1225). Exaptations effectively differentiate the historical origin of a trait and its current function, locating the contingency of evolutionary development in selective events that repurpose a given trait. Mivart’s critique helped Darwin recognize the importance of such examples of exaptation both as a way to explain ‘incipient’ adaptive structures and as a way to underline the essential contingency of natural selection. In light of Mivart’s critique, the exaptive development of plant behaviour took on a further significance, not recognized in Gould’s analysis. If exaptation allowed plants to develop animal-like behaviour, moving as well as reacting to their environment, this showed that complex behaviours could emerge contingently by repurposing traits to serve new functions. Yet the convergence of plant and animal behaviour also demonstrated that complex adaptations could be achieved by radically different exaptive pathways. Darwin’s long-standing interest in the analogy between plant and animal life took on enhanced importance in demonstrating the unexceptional as well as contingent evolution of animal behaviour. Demonstrating the agency of plant life was the linchpin of this analysis because it drew attention to both the complexity of plant behaviour and its analogy with animal action. For the rest of his career, Charles Darwin would doggedly pursue this strategy, working to prove, first, that plants exhibited forms of agency, second, that these behaviours could be explained as the exaptation of traits that did not originally serve their present purpose, and, finally, that these mechanisms were distinct from the (equally contingent) adaptations undergirding animal behaviour.
After making these revisions to On the Origin of Species, Charles launched a series of studies to solidify his argument that plants not only moved, but that this movement was a purposeful behaviour exapted from previously existing traits. This required the development of a variety of new experimental techniques for registering both the contingency of plant behaviour and the contingency of their evolutionary history. Working with his children, Francis, George and Horace, he produced a considerably revised, second edition of Climbing Plants that nearly doubled its length (Reference DarwinDarwin 1875b) as well as an in-depth study of the carnivorous behaviours of Insectivorous Plants (Reference Darwin1875a). These works stunned botanists by showing that virtually all plants exhibited some movement, not just growth, in response to their environment. One outcome of his study of insectivorous plants demonstrated, via various chemical and electrical experiments, that plants possess what he termed ‘nervous matter’, distinct from the nerve tissue of animals (Reference DarwinDarwin 1862a).
At the time, studies of plant physiology were growing considerably more sophisticated. In part, this was due to rigorous new techniques developed by Julius Sachs in his lab at the University of Würzburg. Sachs’s ‘auxanometer’, which mechanically registered plant growth, is one example (Figure 7.2a). Although the auxanometer provided a precise way to study plant growth, it did so with a significant limitation: it could only register growth monotonically along one dimension. Sachs believed that all of the processes that controlled plant movement and growth were rooted in the direct impact of external factors like light, humidity and temperature on the physics and chemistry of growth. This ‘mechanics of growth’, he argued, would eventually explain apparently ‘discontinuous variations of growth’ as the interaction between different continuous processes (Reference SachsSachs 1887: 552). The auxanometer expresses this understanding of plant growth, carefully measuring the vertical growth, normalized as monotonic movement along a single axis, in order to disentangle the influence of various factors. When plotted alongside controlled changes in temperature, humidity or illumination, Sachs believed that the auxanometer would reveal that apparent changes of behaviour were not contingent, irregular events, but rather the unfolding of basic physio-chemical processes.
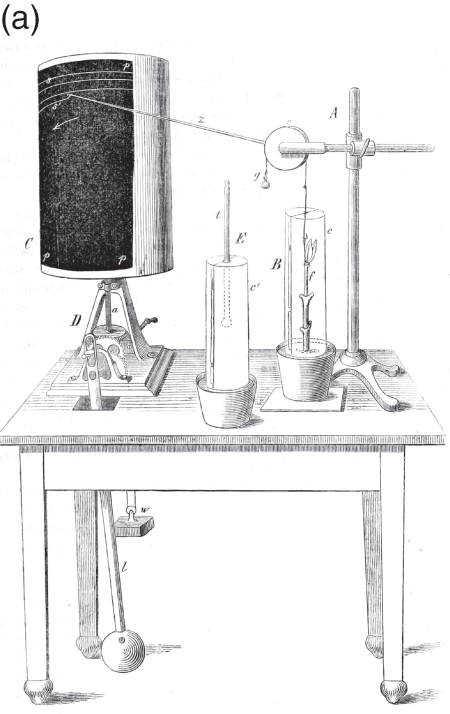
Figure 7.2a Auxanometer.

Figure 7.2b Horace Darwin’s self-recording auxanometer.
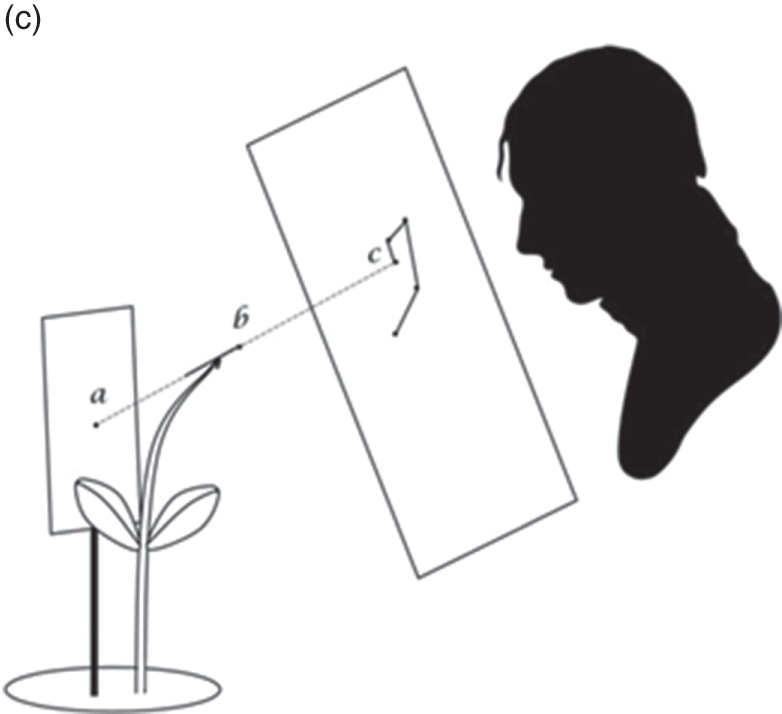
Figure 7.2c Experimental design for Charles Darwin and Francis Darwin’s plant nutation observations
Walter Bryce Gallie and various literary theorists have argued that events are significant to a narrative if they are both non-deterministic and have consequences for later events, affecting the outcome of the narrative (Reference GallieGallie 1964; Reference BarthesBarthes 1975; Reference ChatmanChatman 1978). As Beatty summarizes the distinction, meaningful narratives have ‘turning points’ that are defined both by their temporal and causal relation to later events (the way later events are contingent upon their outcome) and because turning points are contingent per se (they are not necessary, and might have gone some other way) (Reference BeattyBeatty 2016: 36–37). In such cases, as Mary S. Morgan explains, time serves as a ‘material in which we see the dependency of relations or the unfolding of events’ (Reference MorganMorgan 2017: 87). Insisting, by contrast, that plants react in a strictly deterministic fashion, Sachs insisted that plant movement was not contingent per se. As a parallel example of a non-eventful, and so non-narrative, sequence of events, Reference MorsonGary Saul Morson (2003: 61) imagines a description of the movements of Mars that records only where the planet was in each subsequent month, ad infinitum. Such accounts, as Morgan points out, are merely ‘chronicles’: they ‘order events through time’, but do not seek to explain ‘the relations between them’ (Reference Morgan2017: 86). In a similar fashion, Sachs’s interpretation of plant recordings translated the seeming eventfulness of plant growth into the continuous action of physical processes, demoting the narrative of plant behaviour into a chronicle of plant response not essentially different, if seemingly more complicated, from the way planets respond to the interplay of gravitational forces.
Darwin’s studies of exaptation were designed to underline the narrative rather than simply chronological character of evolutionary explanations by making room for the agency of the plants – helping them to function as narrators of their own story by allowing them to record their active response to their surroundings. Impressed with Sachs’s technique, Charles initially asked his son Horace to make a replica of Sachs’s instrument (Figure 7.2b; Horace was an accomplished instrument-maker), and he helped his son Francis secure an invitation to study with Sachs in his lab. Yet they soon abandoned the auxanometer, developing alternative techniques that gave the plants greater freedom of movement. Charles had first begun to try and record their movements in ‘On the Movements and Habits of Climbing Plants’ (Reference 162DarwinDarwin 1865). Placing a hemispherical glass over the tendril and plotting its revolving movement over the course of one workday using a pencil, he confirmed Henri Dutrochet’s earlier studies of the ‘circumnutation’, or revolving movement, of pea tendrils and demonstrated that this rotation sometimes reversed (Reference 162DarwinDarwin 1865: 65). But he lamented that he could not affix the pencil to the plant itself, allowing it to draw its own movement more accurately. Fifteen years later, Charles and Francis announced a breakthrough: they finally devised a scheme to get plants to draw. After smoking glass plates to deposit a layer of carbon, they suspended them at an oblique angle beneath germinated seeds, allowing the small root stems or ‘radicles’ to trace a pattern as they moved across the plate, seeking soil (Figure 7.1).
Taking the pencil out of their own hands and so allowing the plants to trace their own course permitted the Darwins graphically to capture not only the waving path of the roots but variations in force as the tips bent towards and away from the inclined plates. The varying thickness of the line traced by the root tips marks fully contingent narrative events in which the actor (here, the root tips), confronted by an obstacle (the slide), attempts to overcome it. The eventful and non-monotonic nature of each track is underlined by an accompanying textual narrative, which emphasized that ‘Their serpentine courses show that the tips moved regularly from side to side; they also pressed alternately with greater or less force on the plates, sometimes rising up and leaving them altogether for a very short distance’ (Reference BurkhardtBurkhardt et al. 2019: 29). As Francis privately noted, the fact that the tips of the plant roots only lightly touched the plates, rather than ‘pressing hard’ on them, suggested that plants sensed the obstacles and tried to move around them, like hands feeling in the dark (Reference BurkhardtBurkhardt et al. 2019: 27). This thoroughly contingent action is what discriminates these root tracings from mindlessly deterministic behaviour, distinguishing the former as micro-narratives that, per Bal’s definition, describe a series of ‘related events that are [both] caused [and] experienced by actors’.
The various experiments performed by the Darwins on root tips showed a complex form of agency that actively responds to and discriminates between various kinds of stimulus, including light, moisture, physical pressure and the pull of gravity, in order to decide the course pursued by the plant ‘in penetrating the ground’ (Reference Darwin and DarwinDarwin and Darwin 1880: 573). In translating these graphic narratives into text, the Darwins rearticulated the sinuous narrative inscribed by the root tips upon the glass plates, characterizing them as a sequence of turning points, significant events in which the root tip, acted on ‘simultaneously’ by ‘two, or perhaps more, of the exciting causes’, effectively changed its mind, pursuing one course rather than another (Reference Darwin and DarwinDarwin and Darwin 1880: 574). For this reason, the radicle provided the primary evidence that plant behaviour was contingent per se. They concluded that such tracks demonstrate the agency of the plant root:
It is hardly an exaggeration to say that the tip of the radicle thus endowed, and having the power of directing the movements of the adjoining parts, acts like the brain of one of the lower animals; the brain being seated within the anterior end of the body, receiving impressions from the sense-organs, and directing the several movements.
Sachs forcefully rejected the analogy between plant and animal cognition, complaining that ‘Charles Darwin and his son Francis […] on the basis of experiments which were unskilfully [‘ungeschicht’, or clumsily] made and improperly explained, came to the conclusion, as wonderful as it was sensational, that the growing point of the root, like the brain of an animal, dominates the various movements in the root’ (Reference SachsSachs 1882: 843; Reference Sachs1887: 689). The debate between Sachs and the Darwins over the status of plant behaviour – whether plants have the capacity to ‘direct’ their movements – turned on this question: do plants have the capacity to make meaningful changes in the course of their lives; in other words, are they narrative agents? Sach’s auxanometer provides a signal example of the nineteenth-century turn towards ‘mechanical objectivity’, which asserted that increasingly sophisticated instrumental recordings would allow nature to speak for itself (Reference Daston and GalisonDaston and Galison 2007). And yet, any scientific apparatus makes assumptions about a phenomenon under study. Even as such self-recording instruments were designed to produce a neutral or ‘universal’ language of nature, as Soraya de Chadarevian explains, ‘they exerted a normative power on “nature” itself […] forc[ing] the phenomena to inscribe their movements on paper’ in the restricted terms furnished by the apparatus (Reference de Chadareviande Chadarevian 1993: 290). Conversely, the relative looseness of the Darwins’ unmechanized experimental set-up is precisely what allowed plants to demonstrate their wider degree of agency, narrating their own stories.
If Sachs wanted his plants to behave, marching with regular, lawlike action, Darwin wanted his plants to dance to their own tune. Insisting on the analogy between plant and animal agency, the Darwins narrowed the distance between the agency of the scientists and the agency of their object of study. Their smoked glass experiments provide a robust example of the methodological adjustments that Pickering has described as a dance of agencies, in which the human agency of the scientists continually adapts the experimental protocol in order effectively to frame the ‘material agency’ of the phenomena being studied (Reference PickeringPickering 1995: 103). As used by Pickering, ‘dance’ furnishes a metaphor that captures how scientists observe and then actively adjust the experimental protocol when physical phenomena fail to behave in the expected manner (the scientist leads).
The Power of Movement in Plants is striking, in part, because it insists that plants have agency, too – that within the dance of agencies, plants can lead the scientist. This dance is evident in its teeming illustrations of plant movement. The movement of really large plant structures had long been clear. But the movement of tiny shoots, stems and roots was generally so minute it had gone unnoticed. The problem was to connect the human scale to the plant scale. While they remained ignorant of the mechanisms underpinning plant sensation, the Darwins had considerable success recording the mechanisms that underpinned the various forms of plant movement itself. To each structure of interest, they glued a small glass filament, with a bead of wax at the end. Behind the filament, they staked a card with a black dot as an index. And on the other side, they placed a pane of glass perpendicular to the filament, measuring the distance between all three. As the filament moved, they used ink to trace the alignment between bead and index on the pane of glass, taking note of the time (Figure 7.2c). The whole movement was magnified up to thirty times by the differential ratio between bead, index and pane of glass (AB, AC). The result was nearly two hundred illustrations of plant movement that ranged across the gamut of vegetal life.Footnote 2 Take the illustration given in Figure 7.3a, an observation of a fava bean leaf, which captures two days in the life of this plant in the Darwin household. To make each observation, the Darwins had to move with the plant; aligning plant structure, environmental index, glass, pencil, hand and body, at specific moments in time. Individual dots mark observations, moments at which one of the Darwins hovered in alignment with the filament and index card, and marked their line on the glass. Solid lines connect sequential observations; dotted lines indicate periods overnight when the Darwins slept.
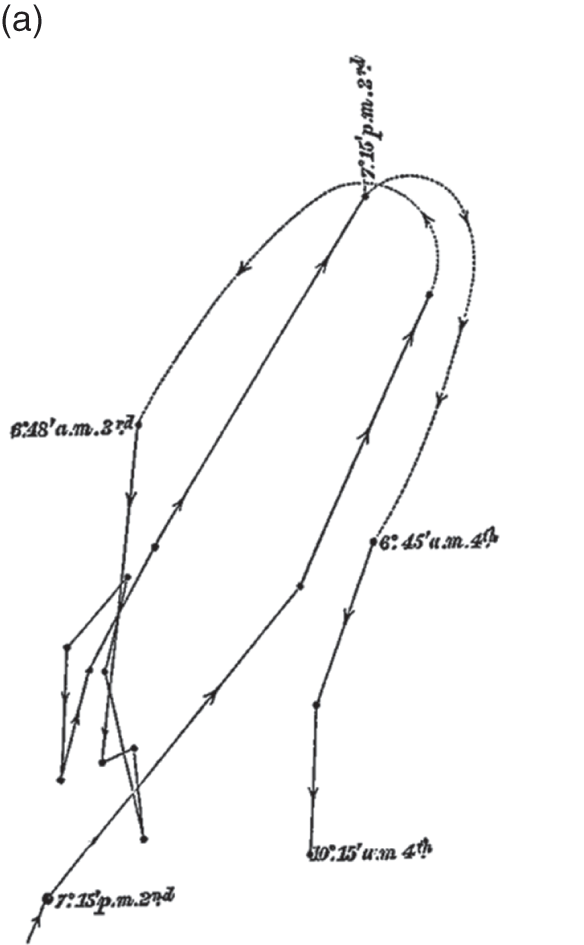
Figure 7.3a Vicia faba
‘Circumnutation of leaf, traced from 7.15 p.m. July 2nd to 10.15 a.m. 4th’ (woodcut).

Figure 7.3b Brassica oleracea
‘Conjoint circumnutation of the hypocotyl and cotyledons during 10 hours 45 minutes’ (woodcut).
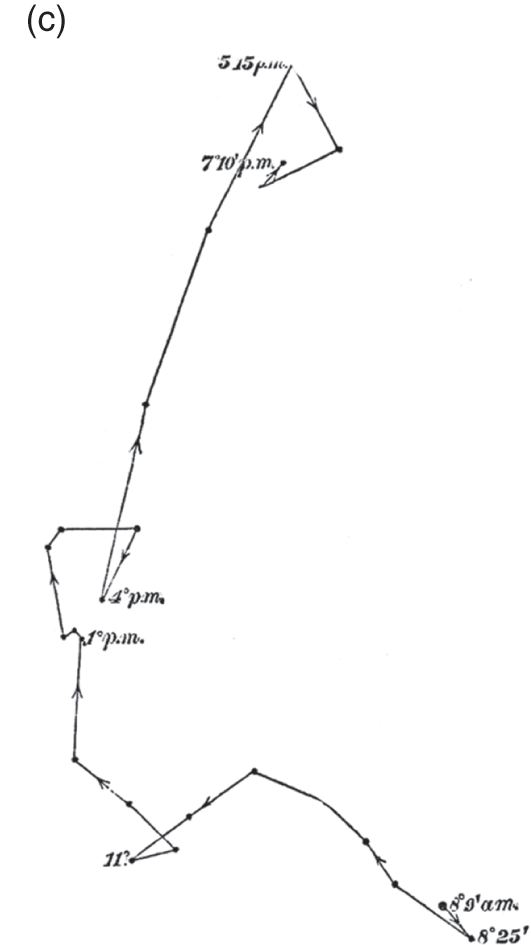
Figure 7.3c Brassica oleracea
‘Heliotropic movement and circumnutation of a hypocotyl towards a very dim lateral light, traced during 11 hours, on a horizontal glass in the morning, and on a vertical glass in the evening’ (woodcut).
In attending to the embodied situation of these experimental events, I take a note from the field of performance studies, which, as Barbara Kirshenblatt-Gimblett explains, emphasizes ‘practice and event [as] a recurring point of reference’, focusing attention on questions of ‘presence, liveliness, agency, [and] embodiment’.Footnote 3 Performance studies furnishes a strategy for reading experimental histories, in the spirit of John Dupré and Daniel H. Nicholson’s ‘Manifesto for a Processual Philosophy of Biology’, as embodied engagements with nature and its ‘hierarchy of processes, stabilized and actively maintained at different timescales’ (Reference Nicholson, Dupré, Nicholson and DupréNicholson and Dupré 2018: 3). Note the synchronies of the interaction between the Darwins and their plants. To make this alignment work, a series of different temporalities have to come into each other’s sway; from stable processes of the physical apparatus (the relative stability of the environment, index card and glass slide, the quick-drying varnish that secured the filament to leaf, the mutability of ink); to the different rhythms of the living agents drawn together by that apparatus. Each slide traces this drama of bodies in motion. Far from clumsy, each mark, each plate, captures another step in an extended attempt – stretching over multiple decades – to learn how to dance with plant life, how to follow its lead.
7.3 Genre and the Reconfiguration of Narrative Levels
The simplicity and sensitivity of the experimental design proved to be its virtue, allowing the Darwins to show that virtually all plant structures moved, and allowing plants to expose their quivering, wakeful life to human view. The Power of Movement in Plants demonstrated the near universality of circumnutation (circular plant movement) across plant species, and across the parts of the plant, from roots and shoots, to leaves and petals, to branches and trunks. Using careful microscopic work, the Darwins also verified that plant movement was produced by the combined action of two traits – variations in the growth of cells on opposite sides of the supporting structure, and more specialized plant structures called ‘pulvini’, in which cells on one side or the other could periodically expand or contract (Reference Darwin and DarwinDarwin and Darwin 1880: 113–116). This, in turn, allowed them to track how circumnutation had been exapted to serve various new functions. They traced myriad examples of heliotropism and apheliotropism (bending towards or away from light sources), geotropism (growing towards the earth) and reactions to temperature and other stimuli.
An important example of this strategy is given in their exploration of Brassica oleracea, or cabbage plant. When we first encounter cabbages in The Power of Movement in Plants, the Darwins document how the seedling, despite lacking a pulvinus, rotates clearly from its base early in its growth, providing a fine example of circumnutation (Figure 7.3b). Later in the study, they return to these seedlings to demonstrate how that behaviour is bent towards different purposes. Moving a similar cabbage seedling near a partially veiled window in the morning they observe how its rotation is deflected and elongated in the direction of the light, only returning to its more circulate rotational behaviour after sunset, at 5.15 p.m. (Figure 7.3c). The strong linear movement from the bottom right to upper left corner of the pane in Figure 7.3c provides a ‘striking’ contrast, they note, to the orderly rotation of Figure 7.3b. Various comparisons like these demonstrate how circumnutation has been adapted to serve a variety of functions, moving towards and away from light, towards and away from gravity, and responding to touch.
In this way, each vacillation in plant movement is tied to a swerve in that plant’s evolutionary history. The extraordinary number of illustrations – five times as many illustrations in a single volume than included in any of Darwin’s other works – demonstrate the variety of events that constitute a plant’s life, and the variety of ways different plants might respond to them. In each, the periodicity of circumnutation provides a background pattern, an elliptical expectation of behaviour that casts any deviation into sharp relief. The Darwins chart deviations in the size, direction and periodicity of these ellipses through the study – the term ‘ellipse’ is itself used nearly two hundred times. Against this elliptical expectation, any sharp deviation of plant movement stands out as a clear fork in the road, the marked reaction of the plant to some stimulus. To put this differently, the ellipse functions in these images as a kind of narrative scaffold, a generic pattern that highlights concrete and consequential events in the narrative.Footnote 4
In essence, each illustration, with its swerves and turns, magnifies a micro-narrative, or better, a micro-history, co-written by the Darwins and their plant subjects. Yet these events do not only mark consequential happenings in the life of the plant; they also index turning points in the evolution of plant life, past moments when circumnutation was exapted to serve a new function. The larger argument set out in The Power of Movement in Plants depends on a multipart analogy between these micro-histories of individual plants, detailed through both their self-inscription and accompanying textual account, and the evolutionary narratives of species history, an analogy that reads differences in the behaviour of individual species as distinct histories of exaptation and adaptive refinement.
For most of the study, over the hundreds of accounts of the growth and movement of individual plants, this analogy is implicit; the authors generally seem to rely on their audience’s knowledge of the wider evolutionary argument of all of Charles Darwin’s studies. This coy positioning of evolutionary argument is abandoned in the conclusion, which gathers all of the observations into a single, unified story. In the final chapter, the study’s scientific narrator draws the various plant micro-histories together, asking that ‘we will in imagination take a germinating seed, and consider the part which the various movements play in the life-history of the plant’ (Reference Darwin and DarwinDarwin and Darwin 1880: 548). The speculation that follows traces a generic seedling from germination, through various events, to its ultimate flowering as a tree – summing up the life events typical for plants in general. The result is a 27-page novella (or mini-novel) that gathers the various experiments explored over the course of the study and organizes them into a unitary narrative that strings together various ‘adaptive movements’ (Reference Darwin and DarwinDarwin and Darwin 1880: 551). Throughout the passage, this narrator slips into present-tense, active formulations that emphasize the plant’s agency, as when ‘our seedling now throws up a stem bearing leaves’ (Reference Darwin and DarwinDarwin and Darwin 1880: 558). When we look at a tree, we see a solid object tossed by the wind, but, in fact, ‘each petiole, sub-petiole, and leaflet’ quivers with activity, activity that marks its continuous reaction to the light, moisture, gravity and other stimuli of its surroundings. Reviewing all the actions that constitute a tree’s life, the narrator comments, ‘All this astonishing amount of movement has been going on year after year since the time when, as a seedling, the tree first emerged from the ground’ (Reference Darwin and DarwinDarwin and Darwin 1880: 558). All of the illustrations of the book, from the elaborate dance steps of the inked filament tracks to the tracings of rootlets on smoked glass, are organized through this single tree’s story, which takes seed and blooms in the mind’s eye.
The ‘life-history’ serves a key function in mediating between the micro-histories of individual plant growth and evolutionary history – a relation set out in Table 7.1. Like David Copperfield or Great Expectations, the ‘life-history of a plant’ gathers various micro-histories and observations into an unfolding story within which an individual actor confronts various challenges and successfully overcomes them. In essence, the Darwins repurpose the Bildungsroman (the contemporary ‘novel of development’ that dominated early to mid-nineteenth-century fiction) as a scaffold capable of interpolating these micro-histories into a single compelling narrative. The accession of this new generic model is marked by specific shifts of narrative point of view (or ‘focalization’), as well as tense, character of action and agent.Footnote 5 All underline a shift from contemporary conventions of scientific monographs. Over the last several centuries, scientific prose has come to rely on passive constructions that minimize the focalization of the scientist–narrator.Footnote 6 And for much of The Power of Movement in Plants, the Darwins similarly deploy passive constructions that describe the experimental design paired with simple-past descriptions of what the plants did. By contrast, the first-person plural ‘we’ that narrates the ‘life-history’ ripples with personality, even as it draws the reader into the act of imaginative engagement. It also facilitates a periodic shift into possessive constructions within the recounting of the ‘life-history’ (including ‘our seedling’), which suffuse the narrative with a sense of familiar responsibility maintained between the narrator’s description, the reader’s implication and the seedling itself, who is now recognized not as an individual scientific specimen but as a more charismatic agent – effectively, our hero. A shift in tense, from the past-tense constructions of the micro-histories to a present-tense unfolding of life events similarly marks a shift in temporal relations and in the character of the events narrated. As many narrative theorists have pointed out, the time of telling and the original timing of the events described in a narrative can never precisely align in either speed or duration (Reference Wittenberg and GarrettWittenberg 2018: 14–15). The ‘life-history’ exacerbates this contrast to powerful effect, dramatizing the distillation of entire life cycles, abstracted across various species, into a handful of crisply plotted pages. The accession of the Bildungsroman structures this turn towards holist integration, centring the account on a unitary actor, sequence and perspective, reconfiguring the events studied throughout the treatise as a series of developing chapters in the life of an individual plant.
Table 7.1 Narrative levels in Charles Darwin and Francis Darwin, The Power of Movement in Plants (Reference Darwin and Darwin1880)
The ‘life-history’ dramatizes the status of plants as active agents within their own narratives. This dramatization, in turn, calls attention to the links between the demonstrated actions of plants, with their various powers, and the antecedent action of natural selection, through which adaptations (especially exaptations) shaped these behaviours and the development of each species. The intermediate status of the ‘life-history’, as the middle stratum of three narrative levels, is made explicit at its close, which situates its story between the micro-histories traced by individual plants and an overarching narrative of species evolution. Listing the various forms of movement studied over the course of the preceding pages, the Darwins assert that ‘it has now been shown’ that these ‘important classes of movement all arise from modified circumnutation’, and they finally, and explicitly, identify the power which has given this plant its various abilities: these uses of the ‘power to bend […] might gradually be acquired through natural selection’ (Reference Darwin and DarwinDarwin and Darwin 1880: 569–570).
If, as Ian Duncan argues (Reference Duncan2019), the Bildungsroman emerged alongside the birth of modern anthropology as a formal model through which an individual life could register the larger story of the human species, the narrator of The Power of Movement in Plants uses their own ‘life-history’ to set out an analogous story about the longer evolution of all plant species. Morgan describes narrative ‘configuration’ as a process that ‘make[s] things cohere – a process of […] making an account that is consistent with all the evidence, that offers a coherency within that account, and that has some explanatory credibility’ (Morgan, Reference Morgan2017: 93). Narrative genres like the Bildungsroman are powerful aids, as they can provide shared standards for the consistency, coherence and credibility of an account. But the ‘life-history’ also demonstrates the role that genres play in reconfiguring previous narratives. Mediating the plant’s relation to evolutionary time, the ‘life-history’ reshapes the micro-histories it draws upon. It is worth thinking more about the role literary genres play in structuring scientific narratives, insofar as they provide such patterns of structure, and establish a ‘horizon of expectations’, that, in Hans Robert Jauss’s influential account, conditions how readers interpret narratives (Reference JaussJauss 1970). Reviewed with the evolutionary implications of the ‘life-history’ in mind, and so, tacitly reconfigured and placed in relation to a wider story, each individual illustration does not simply present the tale of a few hours in the life of an individual plant, but also a concretized retelling of a longer evolutionary history. In light of Darwin’s science, each trait, each action, is pregnant with a history of evolutionary change.
7.4 Conclusion
The dynamic, interactive narratives of The Power of Movement in Plants suggest that we should pay less attention to discrete narratives than to the relation that obtains between various narratives and their world and especially to narration as a process that mediates between a description of events and events in a world, setting them into relation. If narrative, including graphic narratives, do not simply capture, but coordinate events, this means their effect depends on the specific way they coordinate wider patterns, as well as the various purposes to which they are later applied. Perhaps most scientific narratives work this way. Certainly, narratives help scientists to identify and respond to – to sync up with – temporal patterns in the world, and so to coordinate their inscription into the scientific record. Narratives have a peculiarly powerful ability to draw us into an alignment with the world, to train our attention on patterns of action and exceptional events. If we usually think of scientific narratives as perspicuous fictions, ‘designed in the mind’ to model aspects of the world, the unusually active role that plants play in telling their stories within The Power of Movement in Plants suggests that scientific narratives are in part produced by, rather than simply applied to, the world they describe – that the dance of agencies is also a dance of authorships.
Richard Bellon has recently observed the profound influence that Darwin’s plant narratives had on a generation of plant ecologists to come, encouraging them to attend to the sociability of plants and their intertwined evolutionary histories (Reference BellonBellon 2009). Michael Marder, taking stock of the wealth of recent studies that have built on the pioneering work of the Darwins to explore the ability of plants to interact with their environment, and even communicate with each other, underlines their core implication: we need to understand plants as ‘not only a what but also a who, an agent in its milieu’ (Reference Marder and MarderMarder 2016: 42). Plant narratives mediate, pulling our attention, entraining our thoughts, bringing us into contact with nature. In this way, Darwin’s studies, which draw naturalist, specimen and world into a delicate movement, continue to test the conformations of interspecies relations, the anthropology of the inhuman.Footnote 7











