Book contents
- Frontmatter
- Contents
- Dedication
- Preface
- 1 Emerging medical technologies: high stakes science and the need for technology assessment
- 2 Bioengineering and technology assessment
- 3 Health and economic data: a global comparison
- 4 World health and global health challenges
- 5 Healthcare systems: a global comparison
- 6 Healthcare costs vs. time: trends and drivers
- 7 The evolution of technology: scientific method, engineering design, and translational research
- 8 Prevention of infectious disease
- 9 Ethics of clinical research
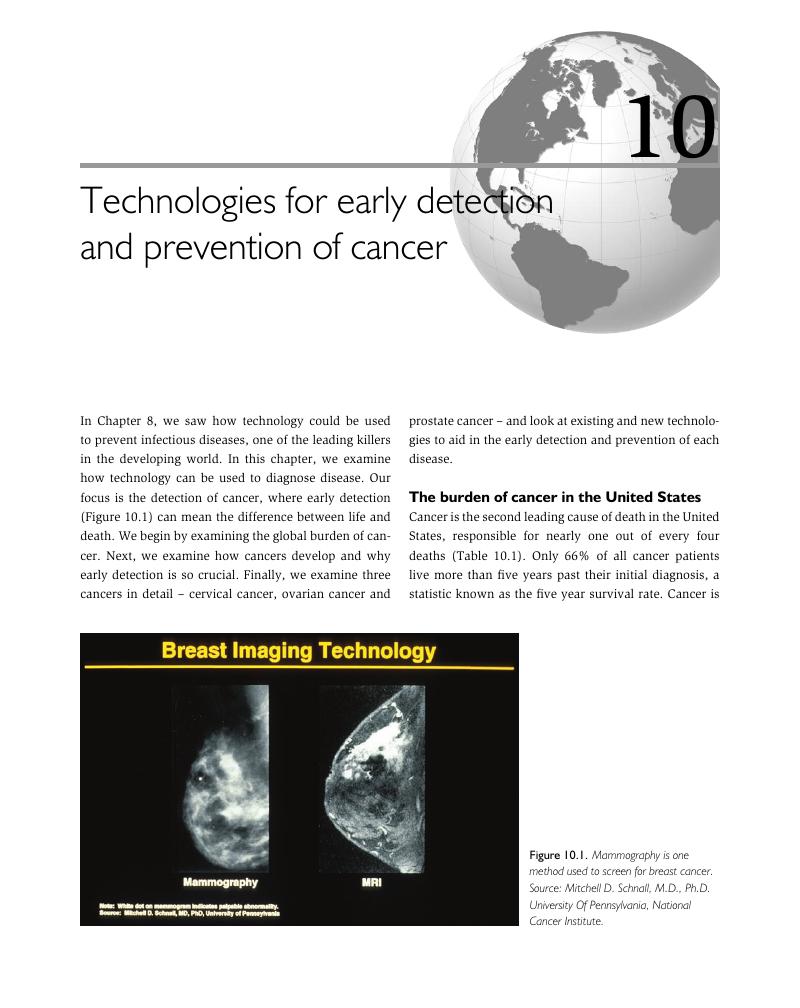
- 10 Technologies for early detection and prevention of cancer
- 11 Cost effectiveness of screening for disease
- 12 Technologies for treatment of heart disease
- 13 Clinical trial design and sample size calculation
- 14 Technology diffusion
- 15 Regulation of healthcare technologies
- 16 Future of bioengineering and world health
- Index
- References
10 - Technologies for early detection and prevention of cancer
Published online by Cambridge University Press: 05 June 2012
- Frontmatter
- Contents
- Dedication
- Preface
- 1 Emerging medical technologies: high stakes science and the need for technology assessment
- 2 Bioengineering and technology assessment
- 3 Health and economic data: a global comparison
- 4 World health and global health challenges
- 5 Healthcare systems: a global comparison
- 6 Healthcare costs vs. time: trends and drivers
- 7 The evolution of technology: scientific method, engineering design, and translational research
- 8 Prevention of infectious disease
- 9 Ethics of clinical research
- 10 Technologies for early detection and prevention of cancer
- 11 Cost effectiveness of screening for disease
- 12 Technologies for treatment of heart disease
- 13 Clinical trial design and sample size calculation
- 14 Technology diffusion
- 15 Regulation of healthcare technologies
- 16 Future of bioengineering and world health
- Index
- References
Summary
- Type
- Chapter
- Information
- Biomedical Engineering for Global Health , pp. 248 - 304Publisher: Cambridge University PressPrint publication year: 2009



