Mild iodine insufficiency and public health
Iodine is essential for the synthesis of the thyroid hormones l-triiodothyronine and l-tetraiodothyronine or thyroxine(Reference Rousset, Dupuy, Miot, De Groot, Chrousos, Dungan, Feingold, Grossman, Hershman, Koch, Korbonits, McLachlan, New, Purnell, Rebar, Singer and Vinik1); iodine deficiency (ID), through impairment of synthesis, can lead to a range of adverse effects, defined in the 1980s as iodine deficiency disorders (IDD). IDD can affect different lifecycle stages with a variety of symptoms, including hypothyroidism, stillbirth, impaired mental function, congenital anomalies and iodine-induced hyperthyroidism(Reference Li and Eastman2). ID is the most preventable cause of brain retardation for the infant(3) and consequences range from loss of intelligence quotient (IQ) to cretinism. The main visible sign of severe ID is goitre.
Impairment of fetal/infant neurological development is irreversible and has lifelong consequences. Neuronal myelination and migration both require thyroid hormones during the early stages of pregnancy and infancy, which depend on iodine availability(Reference Bernal, De Groot and Dungan4). Insufficient intake of iodine during pregnancy can adversely affect both maternal thyroid health (iodine-induced hyperthyroidism or hypothyroidism) and the infant neurological development(3, Reference Leung, Pearce and Braverman5). In its most extreme form, deficiency can lead to cretinism, growth retardation and intellectual impairments, pregnancy losses as well as increased mortality in infants(3). Children born to moderately iodine deficient mothers can have neurological and psychological problems, hyperactivity and decreased IQ scores(Reference Leung, Pearce and Braverman5). In a meta-analysis of both intervention and cohort studies, ID in children aged 5 years and under caused 6·9–10·2 points lower IQ, although high heterogeneity in the evidence selected calls for cautious interpretation(Reference Bougma, Aboud and Harding6). The same study concluded that maternal iodine status is positively associated with infant neurological development and that supplementation with iodine (via intramuscular injection, which is seldom nowadays) appears beneficial in early pregnancy compared with late pregnancy (effect size for mental development d = 0·82). After birth, if the infant is exclusively breastfed, the mother remains its sole source of iodine until weaning, with the offspring potentially exposed to suboptimal iodine levels for at least 13–15 months, 29–33 % of the critical first 45 months of neurodevelopment (depending on weaning age, subsequent complementary feeding)(Reference Bougma, Aboud and Harding6).
Iodine insufficiency, even marginal (urinary iodine concentration (UIC) in population 50–99 µg/l), has been shown to affect children's cognition and their school performance in the UK. The offspring of mothers taking part in the Avon longitudinal study of parents and children had IQ in the lowest quartile (OR 1·58, 95 % CI 1·09, 2·30; P = 0·02) at 8 years when maternal UIC in pregnancy had been below 150 µg/g creatinine(Reference Bath, Steer and Golding7). The use of a single urine iodine measurement only provides a crude categorisation of iodine exposure, which nonetheless resulted in an unexpected outcome for twenty-first century Britain. In epidemiological studies, the median UIC of a population is the commonly used biomarker for the determination of iodine status, as proposed by the WHO(3). Table 1 shows the cut-off points for the categorisation of iodine status based on urine samples, which provides an indication of iodine intake in the short term. Other biomarkers of iodine status measured in blood include thyroglobulin, representative of longer term iodine intake(Reference Ma and Skeaff8), and thyroid-stimulating hormone, which is rarely useful outside of more severe forms of deficiency(Reference Ma and Skeaff8, Reference Bath, Pop and Furmidge-Owen9). While the validation of thyroglobulin as a marker of iodine status is still ongoing (with previously proposed thyroglobulin cut-off for iodine sufficiency of 13 µg/l now understood not to be always applicable)(Reference Ma and Skeaff8), a range of 4–40 µg/l has been described for iodine sufficiency in school-age children(Reference Zimmermann, Moretti and Chaouki10, Reference Zimmermann, de Benoist and Corigliano11).
Table 1. Epidemiological criteria for assessing iodine nutrition in a population based on median and/or range of urinary iodine concentrations(3)

After reports of insufficient status in schoolgirls in 2011 (median UIC 80·1 µg/l, inter-quartile range 56·9–109·0), mild iodine insufficiency in the UK is a renewed public health concern(Reference Vanderpump, Lazarus and Smyth12). Previously believed to be iodine-replete, women in the UK have been shown to be iodine insufficient at the population level(Reference Vanderpump, Lazarus and Smyth12–Reference Lampropoulou, Lean and Combet14). The proposed work in females of childbearing age (cross-sectional survey in Scotland) also established that this population is iodine insufficient (median 75 µg/l)(Reference Lampropoulou, Lean and Combet14). To address this issue in the UK and globally, we must examine the role of awareness, dietary choices and public health strategies.
Dietary choices and iodine intake
Dietary choices are critical for an adequate iodine intake. The main dietary sources of iodine in the UK are marine fish, seafood, seaweed and dairy products, and their consumption varies among women (Fig. 1)(Reference Bates, Cox and Nicholson Polly Page15). In most countries, the main dietary source of iodine is fortified salt(16). To assess habitual iodine intake with minimal participant burden, a short FFQ was previously developed and validated(Reference Combet and Lean17). We found that 60 % of pregnant women do not meet the 250 µg/d WHO recommended iodine intake in the UK(Reference Combet, Bouga and Pan18). Many believe that iodine status is potentially compounded by the consumption of cruciferous vegetables and soya products (collectively known as goitrogenic foods); evidence in human subjects is weak. In a cross-over intervention in healthy women of childbearing age with low habitual iodine intake, we did not find differences in thyroid function in women with increased intake of those foods(Reference Bouga, Cousins and Lean19).
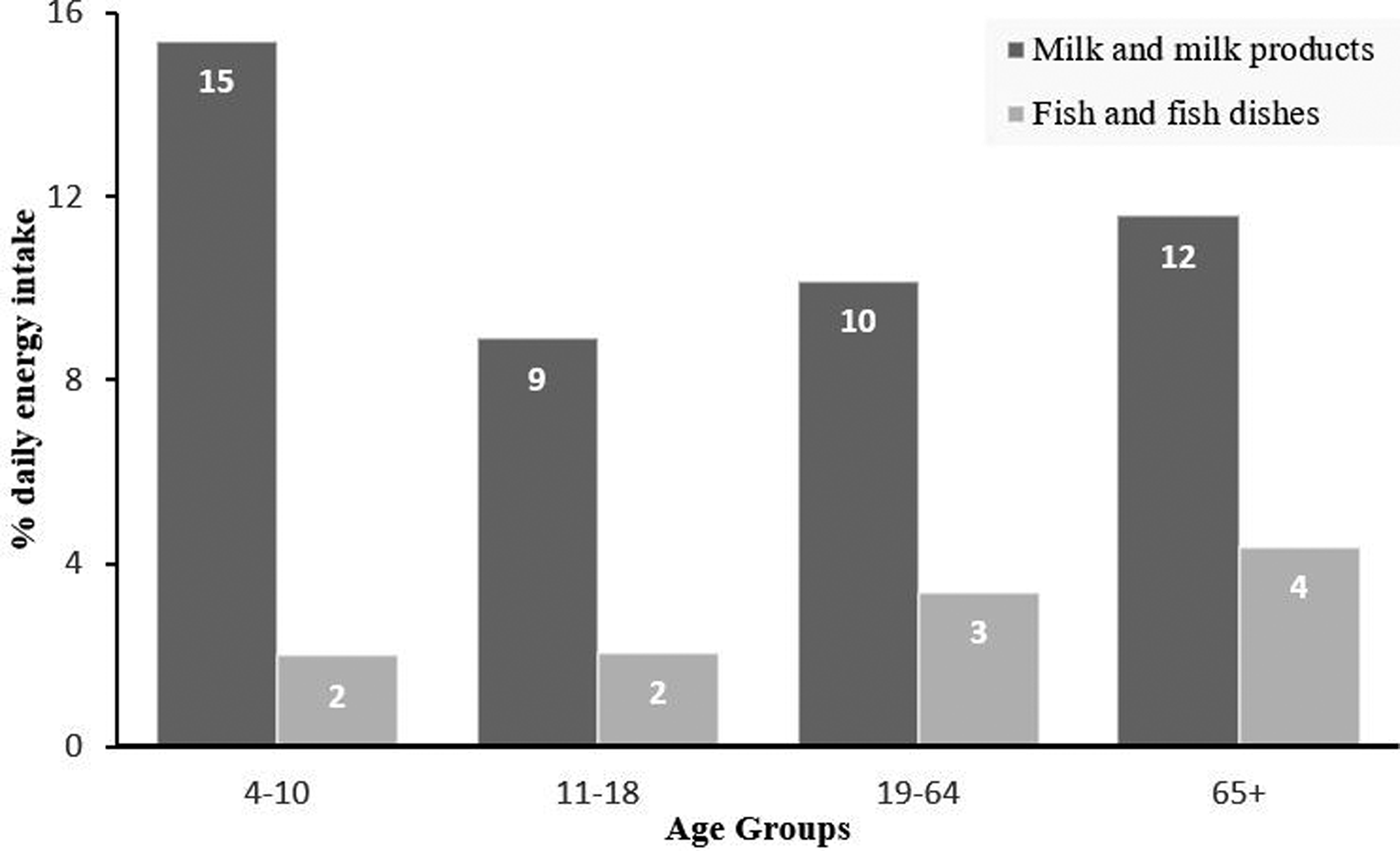
Fig. 1. Iodine-rich foods percentage contribution to daily average total energy intake in women in the UK, based on their age group, based on the National Diet and Nutrition Survey Rolling programme (years 5–6)(Reference Bates, Cox and Nicholson Polly Page15).
Milk and dairy products
We and others have shown that milk is the main dietary source of iodine in the UK(Reference Bates, Cox and Nicholson Polly Page15). Milk and milk products contribute to 38 % of the iodine intake(Reference Henderson, Gregory and Swan20) in non-pregnant adults. In lactating and pregnant women, milk alone contributes towards 38 and 40 % of the dietary iodine intake(Reference Bouga, Layman and Mullaly21). Meanwhile, in pregnancy, iodine is also provided by another dairy (31 %)(Reference Combet, Bouga and Pan18).
Iodine in milk naturally occurs in small levels, and most of the iodine in milk comes from indirect fortification through animal feeds and iodine-containing antiseptic use. Seasonality and farming practices affect milk iodine concentration (ranging from 152 to 256 ng/g), and summer and organic milk have been found to have lower iodine compared with winter and conventional milk (organic milk 26–42 % lower than the conventional)(Reference Bath and Rayman22, Reference Bath, Hill and Infante23). Processing can also affect iodine; ultra high-temperature milk has 30 % lower iodine compared with conventional milk, although the milk fat content has no effect(Reference Payling, Juniper and Drake24). Plant-based milk alternatives do not contain iodine naturally and are rarely fortified, resulting in very low-iodine concentrations; 3·1 (sd 2·5) μg/250 ml; approximately 2 % of the iodine content of conventional milk. Long-term consumption of non-conventional, non-cow's milk can place individuals at risk of iodine insufficiency(Reference Bath, Hill and Infante23, Reference Ma, He and Braverman25).
The current UK recommendations for dairy products intake lack specificity in comparison with the recommendations provided by other countries (e.g. USA, New Zealand, Japan, Australia), which have set easy-to-use portion size guidance for dairy intakes. The recommendation in the updated eatwell guide is to ‘have some dairy or dairy alternatives (such as soya drinks); choosing lower fat and lower sugar options’ which does not differ from the previous UK recommendations, although the dairy products part in the depicted form of the new eatwell guide is slightly smaller compared with the previous eatwell plate(26). In addition, the serving size for milk and other dairy products is not specified(Reference Dror and Allen27) with no differentiation in the dairy product type suggested (apart from the recommendation of choosing lower fat and sugar). The inclusion of dairy alternatives to the recommendation is also concerning (as they may be lacking in protein, calcium, iodine, riboflavin and vitamin B12 if not fortified)(Reference Ma, He and Braverman25, Reference Heaney, Dowell and Rafferty28, Reference Sethi, Tyagi and Anurag29).
The identification of barriers, facilitators and perceptions towards iodine-rich foods consumption is important, considering their potential input in increasing iodine intake. Perceptions of healthiness are closely related to dietary behaviour and food choice; attitudes to healthy eating are influenced by factors such as sensorial characteristics, culture, food availability, child feeding and energy density(Reference Carrillo, Varela and Salvador30). Consumer perceptions towards aspects of dairy products have been previously investigated, with their perceived healthiness ranked as ‘relatively healthy’(Reference Johansen, Naes and Hersleth31). Women's perceptions of dairy foods, examined through focus groups, highlighted awareness of dairy high-calcium content and high-fat content. The taste of some products, including low fat, was reported as unsatisfying. Convenience was reported as an important factor, potentially partly compounded by the increased cost of such products. Dairy products, however, are considered as staples in the everyday life of many, and neither cost nor convenience would affect purchasing decision(Reference Hagy, Brochetti and Duncan32). Other drivers of dairy food choices include not only taste but also other family member's preferences and perceived health benefits(Reference Richardson-Harman, Stevens and Walker33, Reference Hammond and Chapman34) as well as sex, age and socioeconomic status, which also determine the acceptance of functional and enriched foods(Reference Ares and Gambaro35).
Fish and seafood
Fish is a rich natural source of dietary iodine, and it is the main contributor to the UK dietary iodine intake after dairy, contributing towards 11 % of the intake in non-pregnant adults(Reference Bates, Cox and Nicholson Polly Page15, Reference Henderson, Gregory and Swan20), and 24 % in pregnancy(Reference Combet, Bouga and Pan18).
The diversity of fish and seafood products creates a variety of food choices with a spectrum of iodine contents. White fish, such as haddock and cod, contains more iodine than oil-rich fish (approximately 48 µg/100 g in oily fish v. 105 µg/100 g in white fish)(Reference McCance and Widdowson36). Iodine also varies within species and decreases from the skin to the inner part of the fish fillets, with levels twenty times higher in the skin of marine species such as cod(Reference Karl, Münkner and Krause37). Marine fish contains the highest amounts of iodine, ranging from 40 to 69 µg/100 g, which is also approximately 6-fold higher compared with freshwater fish(Reference Haldimann, Alt and Blanc38). Cooking can affect the iodine content of fish, with losses varying in average from 20 % in fried fish to 23 % in grilled fish and 58 % in boiled fish(Reference Harrison, McFarlane and Harden39). Other seafood (including prawns, crab, lobster) have an average iodine content of 92 µg/100 g and are also a good source of iodine.
Seafood consumption is important both in pregnancy and in the general population as it provides iodine, as well as n-3 fatty acids, protein and other micronutrients such as vitamin D, vitamin A and selenium. In the UK, the recommendation is to consume two portions of fish per week (2 × 140 g), one of which should be oily(26). According to the National Diet and Nutrition Survey, oily fish intake still remains lower than once weekly(Reference Bates, Cox and Nicholson Polly Page15). White fish is consumed more often compared with oil-rich fish(Reference Whitton, Nicholson and Roberts40). During pregnancy, women should consume at least two portions of fish per week, one of which should be from oil-rich fish. However, simultaneous advice against consuming types of fish with potentially harmful levels of mercury, as well as raw shellfish due to harmful bacteria and risk of poisoning, may create confusion over the recommendation for fish intake.
The theory of planned behaviour, used in the context of intention and frequency of fish consumption in Iran, concluded that the perceived behavioural control and the intention to eat fish predict the frequency of fish consumption (R 2 0·58, F = 223·1, P < 0·001)(Reference Aghamolaei, Sadat Tavafian and Madani41). Whether people include fish in their diet depends on the drivers of food choice. There is a gap between the scientific evidence of risks and benefits related to fish and seafood consumption and actual beliefs and perceptions of consumers(Reference Verbeke, Sioen and Pieniak42). In our interviews with women in the perinatal period, taste and heartburn have been described as the main drivers for inclusion or exclusion of fish, seafood and dairy products in the diet. Fish consumption is only well accepted during pregnancy by <20 % of the population, with taste and smell the main barriers(Reference Bouga, Lean and Combet43). Most believe that eating fish is beneficial for health (94 %; survey of 329 people in the USA with benefits attributable to n-3 oils according to 45 %). A substantial proportion also perceived consumption to be risky (70 %, main risk attributable to mercury content, according to 24 %)(Reference Burger and Gochfeld44). In five European countries, fish consumption was associated with country-related traditions and habits, which outweighed perceptions of risks and benefits(Reference Jacobs, Sioen and Pieniak45). Ethical factors also feature in the choice to consume fish, with Danish respondents willing to pay more for welfare fish than farmed fish (48 %)(Reference Solgaard and Yang46).
Seaweed
Seaweed is a rich source of iodine, suitable for vegan and vegetarian populations. The iodine level in seaweed products if very broad (range 11–6118 µg/g of dried seaweed) and could lead to an iodine excess, beyond the European tolerable upper limit of 600 µg daily. Labelling of seaweed-containing products is generally poor, with limited information on iodine content or seaweed specie on the product packages(Reference Bouga and Combet47). Only 10 % of the seaweed-containing products stated information regarding iodine content, and 18 % enabled its estimation. A total of twenty-six products surveyed could lead to an intake above the adult tolerable upper limit if consumed(Reference Bouga and Combet47). While sushi dishes are reported to be consumed at least once per year by 45 % of the population(Reference Brunstrom, Shakeshaft and Alexander48), they mostly contain Nori seaweed, with a lower iodine content of 16 µg iodine/g (an average sheet of Nori being approximately 3 g)(Reference Teas, Pino and Critchley49).
Iodine knowledge and awareness
The low profile of iodine in the UK public health and media arenas can potentially explain the low knowledge and awareness about the nutrient amongst mothers and healthcare professionals (HCP). Pregnant women receive general dietary guidance during pregnancy, which is usually delivered by the community midwives during the first antenatal care appointment. These recommendations focus on following a balanced diet with limited specific practical recommendations on foods to include/increase/decrease/exclude or portion sizes(50). The first antenatal appointment usually happens around the 12th week of pregnancy, with the content of the discussion varying between cases, dependent on both midwife's and woman's knowledge, education and personal interest in nutrition(Reference Bouga, Lean and Combet43).
Healthcare professionals
HCP have low awareness of iodine in women of childbearing age, its importance, sources and recommendations. Recommendations in the USA include daily prenatal vitamins containing 150 µg iodine during preconception, pregnancy and lactation. Obstetricians and midwives (web-based survey, n 476) recognised (60 %) that supplementation in childbearing age and pregnancy is useful, but most (75 %) reported to rarely or never prescribe iodine-containing supplements(Reference De Leo, Pearce and Braverman51). Australian guidelines also recommend iodine supplementation and although 71 % of the HCP were aware of the recommendation, knowledge regarding the recommended dose and duration was low(Reference Lucas, Charlton and Brown52, Reference Guess, Malek and Anderson53). Iodine supplements were recommended by 73 % of the respondents during pregnancy, but only by about 50 % in preconception and lactation. Reasons to not recommend supplements were the existence of fortification programmes (28 %) and lack of awareness of the recommendation (25 %). The midwives who took part in the survey reported lack of knowledge (40 %) and were less likely than the dietitians to discuss dietary sources of iodine, which only 40 % of the HCP reported discussing with women. In New Zealand, where public health nutrition has focused on iodine for several decades, almost 100 % of healthcare workers (pharmacists, midwives and hospital nurses) reported a high knowledge of iodine supplementation and fortification (but the sample of this survey was smaller (n 25) compared with the rest of the surveys)(Reference Nithiananthan, Carroll and Krebs54). Knowledge has been also associated with the speciality of the HCP in Turkey. Endocrinologists had significantly higher knowledge and awareness on iodine supplementation, duration and iodised salt compared with family practitioners and obstetricians. However, in the same survey, knowledge was very low for all three specialities (endocrinologists, family practitioners and obstetricians) when asked whether supplementation in pregnancy should be recommended during the existence of food and salt iodisation programmes(Reference Kut, Kalli and Anil55).
In the UK, only 46 % of midwives could correctly identify seafood as a source of iodine, and 23 % dairy products (Reference Lucas, Charlton and Brown52); this is not surprising since nutrition is still not a significant part of the curriculum. Most midwives (67 %) reported not mentioning iodine in antenatal care, as only 20 % could link it to fetal development and 10 % were aware of the increased iodine requirements during pregnancy. A need and strong interest for further education on iodine was expressed by the majority of HCP interviewed, focusing on pregnancy, guidelines and sources(Reference Lucas, Charlton and Brown52, Reference Guess, Malek and Anderson53, Reference Williamson, Lean and Combet56).
Women of childbearing age
Globally, both iodine knowledge and awareness are low among women of childbearing age (pregnant, lactating or not). In a cross-sectional survey of 1026 UK mothers, 55 % were unable to identify sources of iodine, commonly mistaking salt (21 %) and vegetables (54 %) as iodine-rich foods. However, most (87 %) reported a willingness to modify dietary behaviour if they received information related to the importance of iodine in pregnancy. In this study, only 9 % of women surveyed could recognise milk as a source of the micronutrient(Reference Combet, Bouga and Pan18). Similar were our findings from the interviews of forty-eight women in preconception, pregnancy and new mothers. Women reported rarely discussing iodine with their HCP, and lacking knowledge of dietary sources and importance of iodine for fetal development(Reference Bouga, Lean and Combet43). In Australia, a country with mild iodine insufficiency in pregnancy and mandatory iodine fortification of salt and bread as well as recommendations for iodine supplementation in pregnancy, knowledge regarding the adverse outcomes of ID and the importance of iodine has been found to be consistently poor in pregnant women(Reference Lucas, Charlton and Brown52, Reference Charlton, Gemming and Yeatman57–Reference Malek, Umberger and Makrides60). Low self-confidence on whether women met the iodine requirements (20 %) could be explained from the lack of knowledge of dietary sources of iodine. Seafood, the most commonly recognised iodine source, was correctly identified by 23–55 % of the women, depending on the survey. However, milk was only recognised as a rich source of iodine by 15–29 % of pregnant women. Almost half of pregnant women mistook vegetables as rich sources of iodine. Finally, supplementation with iodine was not considered necessary by 41 % of pregnant women, dropping to 18·5 % when they followed a diet perceived to be healthy. Knowledge was identified as a predictor of iodine supplementation, and women who thought that the intake of iodine supplements in pregnancy is important, regardless of how healthy a diet they follow, were more likely to take supplements containing iodine(Reference Martin, Savige and Mitchell59). Poor knowledge did not improve after the introduction of the mandatory iodine fortification programme(Reference Charlton, Yeatman and Houweling61). In Iran, similarly, women of childbearing age have low knowledge, awareness and practice in relation to ID(Reference Mirmiran, Nazeri and Amiri62–Reference Nazeri, Mirmiran and Asghari64), which has been linked to lower iodine status(Reference O'Kane, Pourshahidi and Farren65). As a result, increasing awareness and knowledge would be potentially a cost-effective way of increasing iodine intake.
Global prophylactic measures and the UK
The potential level of intellectual impairment in a significant proportion of the population and the net cost to both society and the economy due to iodine insufficiency are important. An iodine-insufficient population poses high healthcare and societal national costs, with iodine supplementation in pregnancy modelled to save £199 in healthcare costs and £4476 from a societal perspective (for an increase of 1·22 IQ points per offspring)(Reference Monahan, Boelaert and Jolly66). To date, there is no public health nutrition programme in the UK addressing this pressing, totally preventable, concern, such as fortification or supplementation. Moreover, dietary recommendations for iodine have not changed since 1991.
Iodine recommendations
The WHO/UNICEF/International Council for the Control of IDD recommended the daily intake for adults is 150 µg/d, increasing to 250 µg/d for pregnant women(3). The European Food Safety Authority proposed in 2014 a new reference value of adequate intake for pregnant women of 200 µg/d(67). The US Institute of Medicine and the Food Standards Australia New Zealand also propose an increase in iodine intake for pregnancy and lactation. However, in the UK, the Department of Health reference nutrient intake is for adults 140 µg/d, with no proposed increment for pregnancy and lactation (Table 2)(68). Iodine requirements vary with age (Table 2), with no sex differentiations in the recommendation, besides from pregnancy and lactation. However, it is now recognised that iodine intake in preconception is important and may impact on neonatal outcomes(Reference Abel, Ystrom and Caspersen69).
Table 2. Existing iodine recommendations (μg/d)
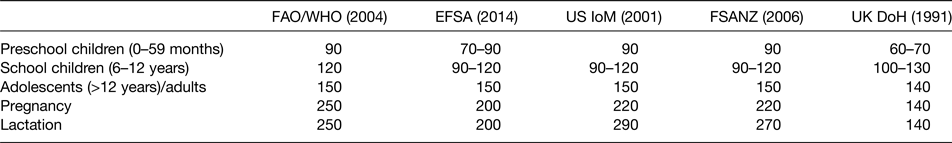
DoH, Department of Health; EFSA, European Food Safety Authority; FSANZ, Food Standards Australia New Zealand; IoM, Institute of Medicine.
There is an ongoing debate regarding the thresholds of sufficiency in pregnancy and the different existing recommendations for tolerable upper limit of intake, which ranges from 600 µg/d(70) in Europe (Scientific Committee on Food) to 1100 µg/d(71) in the USA (Institute of Medicine). A large-scale cross-sectional study in Chinese pregnant women suggested that UIC in pregnant women should not exceed 250 µg/l in iodine-sufficient regions, due to high risk of subclinical hypothyroidism (1·75-fold increase in UIC 250–500 µg/l). UIC exceeding 500 µg/l is also associated with isolated hypothyroxinaemia (2·85-fold increase). Levels of autoimmunity, following a U-shape curve, are lowest in women with UIC 150–250 µg/l. This leaves a potentially narrow margin of sufficient intake, which would be difficult to control around the world, due to the different iodine content of foods, salt and lack of labelling(Reference Shi, Han and Li72). Accordingly, Lee and Pearce(Reference Lee and Pearce73) proposed that the upper level of sufficiency in pregnancy should be an intake of 250 µg/d.
Since 15–20 mg of iodine is stored in the body of a healthy adult (70–80 % in the thyroid), intermittent consumption is acceptable, with thyroid hormone synthesis requiring approximately 60–95 µg iodine daily based on iodine turnover, which is close to the lower reference nutrient intake of 70 µg/d(Reference Zimmermann74). The recommended WHO intake of 250 µg/d could be met by consuming two portions of fish per week, and dairy to the equivalent of two glasses of milk (drinks, in cereals), plus one yoghurt and a cheese serving daily. However, many women avoid these foods and lack guidance on how to include them in their diet.
Universal salt iodisation and fortified foods as potential vehicles
The elimination of ID and related disorders is a priority for the WHO and UNICEF. Universal salt iodisation has been adopted by over 120 countries globally(16). It is the main method of iodine prophylaxis worldwide, first proposed in 1820. First attempt of salt fortification with iodine was done 100 years later(16). The proposed iodisation of salt is 20–40 mg/kg and is based on an average salt intake of 10 g daily. Salt has been chosen as a vehicle of salt iodisation as it combines characteristics that make it suitable, including its stable consumption throughout the year, low cost, consumption by everyone in a population, ease in implementation, quality, odour and taste not being affected and monitoring of production(Reference Venkatesh Mannar and Dunn75).
Salt iodisation is not considered unanimously a good practice for the control of ID and there is still a debate on its success and potential risks, which might contribute to the lack of legislation for salt fortification in the UK. The perceived conflicting messages that universal salt iodisation would convey remains at odds with public health campaign for salt reduction to <5 g/d(Reference Tonacchera, Dimida and De Servi76, Reference Charlton, Webster and Kowal77). Experts from the WHO, UNICEF and International Council for the Control of IDD work together to overcome any counterproductive effects of the two public health campaigns and find a common ground for their parallel success(Reference Webster, Land and Christoforou78). According to the WHO, salt iodisation and salt intake reduction (in <5 g/d) are both important, and there is a need to understand that they can be compatible(79). Iodine fortification could increase in line with the decrease of salt intake and mandatory fortification would remove the positive bias of iodised salt as ‘healthier’(Reference Webster, Land and Christoforou78). Further argument needs to be considered, including (lack of) freedom of choice in the context of mandatory fortification and the risk of high exposure/toxicity for a sub-group of the population.
While IDD have been successfully eliminated or controlled in many countries, via salt fortification in combination with diet diversification (in the USA(Reference Leung, Braverman and Pearce80) and Ghana(Reference Nyumuah, Hoang and Amoaful81), with exceptions in European countries(Reference van der Haar, Gerasimov and Tyler82)), consumption of fortified salt may not be a sufficient measure in pregnancy(83). Studies in Italy(Reference Marchioni, Fumarola and Calvanese84), Turkey(Reference Kut, Gursoy and Senbayram85, Reference Anaforoğlu, Algün and İnceçayır86) and Tasmania(Reference Burgess, Seal and Stilwell87) showed that ID in pregnant women persisted even after the application of universal salt iodisation, with UIC<150 µg/l in 92, 50–78 and 73 % of pregnant women in each country, respectively. Salt fortification with iodine is voluntary in the UK; iodised salt therefore does not contribute to the iodine intake of the population, with restricted availability in the market (weighed availability in market share 21·5 %)(Reference Bath, Button and Rayman88).
Fortification of other foods is also an option, although the International Council for the Control of IDD does not support individual food iodisation(Reference Charlton and Skeaff89). In Bangladesh and Pakistan, fortification of processed foods with iodised salt increased the availability of iodine in the population, and manufacturers use it when legislation permits, as it does not negatively affect the food characteristics(Reference Spohrer, Garrett and Timmer90). Fortification of bread with iodised salt, in Australia, resulted in increased iodine intake in pregnancy (median UIC 124·2 µg/d, inter-quartile range 121·1–127·2) and postpartum (median UIC 123·4 µg/d, inter-quartile range 119·7–127·1)(Reference Mackerras, Powers and Boorman91). The choice of bread was the result of extended modelling for the identification of be the best vehicle for the increase of iodine intake(Reference Charlton and Skeaff89). Recently, biofortification of vegetables with iodine was also proposed as an opportunity to increase iodine intake. Positive results have been published after the consumption of fortified vegetables in fifty healthy volunteers in Italy, with UIC increased by 19·6 % (P < 0·05)(Reference Tonacchera, Dimida and De Servi76). Turmeric can also help in the elimination of goitre in the increase of iodine intake, based on a study in Pakistan. The authors of this study suggest that the use of iodine-fortified salt should not be overemphasised, as alternatives (such as turmeric) could be implemented(Reference Jawa, Jawad and Riaz92), an opinion which is not widely accepted considering the usefulness of iodised salt in the correction of IDD(Reference Elahi, Syed and Saleem93).
A meta-analysis of nine randomised controlled trials (RCT) during 1990–2012 looked at the effect of iodine-fortified foods on UIC of children aged 7–10·5 years. Fortified foods included biscuits, meals and milk and the contained dose of iodine ranged from 25 to 200 µg/l, consumed for 4–30 months. At baseline, the UIC was similar in both the intervention and controlled groups (heterogeneity Q = 942·47, df = 13). No carry-over effect was observed in cross-over trials, so trials with both cross-over and parallel designs were included in the meta-analysis. The standard mean UIC was significantly higher in the fortified group when compared with the control group (standardised mean difference = 2·02, P < 0·001) with iodine-fortified foods effective to improve UIC in children(Reference Athe, Mendu and Krishnapillai94).
It is important to consider acceptance of biofortified foods in populations and their wider production, prior to implementing strategies including these foods. Based on the Protection Motivation Theory, parents and school heads in Uganda were surveyed regarding their reactions to adopting iodine-biofortified staple foods in the school feeding programmes. Knowledge of parents and school heads about micronutrients, IDD and biofortification was low, with iodine and salt iodisation being the only two topics with higher awareness. Conversely, threat appraisal (perceived severity, vulnerability and fear to evaluate ID) and coping appraisal (response efficacy, cost response and self-efficacy to deal with ID through biofortified foods) were high for both sub-samples, which favours the protection motivation. The intention to adopt biofortified legumes was high and depended on factors including cost of the products, age and sex of the respondents. Key aims of a feeding programme should include increased awareness of the health effects of ID and low cost of the biofortified foods(Reference De Steur, Mogendi and Wesana95).
Supplementation in pregnancy
Supplementation is an alternative strategy to address iodine insufficiency in pregnant and lactating women. However, healthy start supplements, provided by the UK health services do not contain iodine, and commercial alternatives are expensive. Similarly to the USA(Reference Leung, Braverman and Pearce80), marketed pregnancy supplements are not required to contain iodine, although their use has been associated with a 40 % higher UIC in Spanish pregnant women(Reference Alvarez-Pedrerol, Ribas-Fitó and García-Esteban96). The American Thyroid Association, the Endocrine Society and the US National Academy of Sciences have proposed that all prenatal supplements should include 150 µg potassium iodide(Reference Leung, Braverman and Pearce80). The WHO also recommends iodine supplementation in pregnancy and lactation in all countries where iodised salt is available in <20 % of the households(3).
A recent Cochrane review of positive and negative health effects of iodine supplementation in preconception, pregnancy and lactation, for the mother, the infant and the child highlighted inconclusive evidence(Reference Harding, Pena-Rosas and Webster97). There was an indication of both harm and benefit in places of mild-to-moderate deficiency. The number of available studies was limited, potentially due to the ethical difficulties implementing studies with a placebo/control group in pregnancy. Potential benefits included lower likelihood of insufficient iodine status in pregnancy, congenital abnormalities, postpartum hyperthyroidism, neonatal goitre and neonatal insufficient iodine intake(Reference Harding, Pena-Rosas and Webster97). Potential harm included overactive thyroid function, nausea and vomiting during pregnancy. A cohort study in pregnant women with mild-to-moderate ID, including women receiving prenatal iodised (150 µg) supplements (n 168), women who regularly used iodised salt (n 105) and a control group of women (n 160), found that thyroid-stimulating hormone was significantly higher in women taking supplements than in the other two groups, and 26 % of women had higher thyroid-stimulating hormone than the upper limit for gestation. Consequently, as mild ID women who take daily a 200 µg iodine supplement from the beginning of their pregnancy might have an increased thyroid-stimulating hormone and risk of maternal hyperthyrotrophinaemia, supplementation with iodine for a long period prior to conception is suggested for women living in mild-to-moderate deficient areas(Reference Moleti, Di Bella and Giorgianni98). Iodine supplementation did not have an effect on thyroid dysfunction in a mild-to-moderate deficient area in Denmark, in thyroid peroxidase antibody-positive pregnant women. Women who participated in a placebo control-led RCT received a daily mineral and vitamin tablet with or without 150 µg iodine (group A: no iodine, group B: iodine during pregnancy only, group C: iodine during pregnancy and postpartum). Postpartum thyroid dysfunction developed in 55 % of the participants, without any difference between the three groups(Reference Nohr, Jorgensen and Pedersen99).
Beside impact on iodine status and thyroid function, the effect of iodine (supplementation) on neurodevelopment is critical and should be the key outcome for the assessment of supplementation efficacy. Iodine intervention studies in pregnancy have measured an actual cognitive outcome in children from 3 months to 5·4 years(Reference Kevany, Fierro-Benitez and Pretell100–Reference Gowachirapant, Jaiswal and Melse-Boonstra108). In India and Thailand, iodine supplementation in pregnancy did not lead to a measurable difference in verbal IQ, performance IQ or the global executive composite score from the Behaviour Rating Inventory of Executive Function Preschool Version, assessed in children at 5·4 years (200 µg daily iodine or placebo during pregnancy)(Reference Gowachirapant, Jaiswal and Melse-Boonstra108). The Spanish multicentre mother-and-child cohort (INMA cohort, Valencia, Sabadell, Asturias and Gipuzkoa areas) in 1519 1-year-old infants showed a lower psychomotor development index score (−4·9 and −5·5 points, respectively) in children whose mothers were taking ≥150 µg/d from supplements compared with children whose mothers consumed <100 µg/d iodine from supplements (Bayley scales of infant development for psychomotor and cognitive development) in the regions of Asturias and Valencia. When the results of all the areas were put together for the comparison of these two groups (≥150 v. <100 µg/d from supplements), a 1·5-fold increase in the odds of a psychomotor scale score <85 was found (which might indicate a slight delay in neuropsychological development) but no difference for the mental development index or UIC(Reference Murcia, Rebagliato and Iniguez105, Reference Rebagliato, Murcia and Álvarez-Pedrerol106). Furthermore, no significant differences in children's neurological development were shown in iodine supplementation studies in pregnant women in Spain(Reference Santiago, Velasco and Muela107) and Australia(Reference Zhou, Skeaff and Ryan109). However, the Australian study stopped without recruiting the required number of participants and the results may be underpowered. A key factor in the interpretation of these studies is the age of assessment since neurocognitive testing is not reliable in the youngest groups.
Severe ID, mainly in early pregnancy, was shown to lead to cretinism in a trial of iodine supplementation through intramuscular injection(Reference Pharoah, Buttfield and Hetzel101). Positive associations between supplementation in mild-to-moderate deficient areas and children's neurodevelopment were shown in Spain. Daily potassium iodide supplement (300 µg/d) in the first trimester led to an increased psychomotor development index score in children (assessed at age 3–18 months)(Reference Velasco, Carreira and Santiago104). Positive results of iodine supplementation in pregnancy (200 µg KI/d) in relation to neurodevelopment have been also found in a study in 18-month-old children born to women with hypothyroxinaemia in early pregnancy(Reference Berbel, Mestre and Santamaria103). Finally, IQ score was 11·2 points higher (95 % CI 7·96, 14·46) in 4–23 months old children of women who received iodine via intramuscular injection during pregnancy (after the prenatal consultation between 20 and 36 weeks of gestation) or delivery; however, those studies were published 40–50 years ago, in areas with severe ID and endemic goitre(Reference Kevany, Fierro-Benitez and Pretell100, Reference Thilly, Delange and Lagasse102).
From those intervention studies, there is overall a neutral or positive impact of supplementation during pregnancy on the neurological development of the infant. However, the reliability of the different assessment methods of neurodevelopment in a very early age might be a potential reason for the non-conclusive results. More well-designed and longer term studies are needed to draw conclusions, assessing neurological development in older children(Reference Zhou, Anderson and Gibson110).
Considerations for the future
The re-emergence of ID in the UK, highlighted in 2011(Reference Vanderpump, Lazarus and Smyth12), is not a new public health concern anymore; however, 60 % of pregnant women still have an iodine intake lower than the WHO recommendation(Reference Combet, Bouga and Pan18). Eating patterns have changed in the past 20 years, with a decrease in milk intake(Reference Whitton, Nicholson and Roberts40), potentially driven by commercial pressures and marketing (e.g. promotion of milk alternatives). Simultaneously, changes have occurred in farming practices, due to thyrotoxicosis from the high levels of iodine in milk as a result of the addition of iodine in cattle feed and use of iodophor disinfectants used in sanitisation(Reference Wheeler, Fleet and Ashley111, Reference Bath, Button and Rayman112). The consequences of ID are not limited to the peri-conception and pregnancy periods, since the effects of ID are often lifelong and irreversible, thereby impacting on society, with decreased productivity and increased costs(Reference Monahan, Boelaert and Jolly66). Prophylaxis via salt fortification is relatively cheap (2–7 US cents/kg, <5 % of the salt retail price)(Reference Mannar, Dunn and Initiative113) but may not be a sufficient measure during pregnancy and lactation. Meanwhile, evidence of the benefits of supplementation is still unclear, and potential impacts on recommendations made by HCP.
ID is a diet-related challenge, and the strategy to tackle this challenge must include public health and policy strategies, without ignoring the role of foods, dietary recommendation and knowledge/awareness. The lack of involvement of diet and nutrition professionals as part of the solution, and the lacking nutrition content of most curriculum for the health profession are likely to blunt the effectiveness of any given strategy and should be re-evaluated. Iodine-rich sources in the diet are varied, and our qualitative study has shown that women of childbearing age are receptive to dietary and lifestyle changes as long as guidance and support is provided, inviting strategies in this area. However, dietary guidance during antenatal care is perceived to be insufficient and confusing, driving women to use other sources of information, sometimes less credible(Reference Bouga, Lean and Combet43). A clear need for empowerment in pregnancy emerges, as women are willing to follow specific and comprehensive dietary advice in pregnancy. Public health strategies and educative programmes could therefore influence the improvement of nutritional status in the perinatal period and an increase of iodine status of the population.
There is very limited evidence on the effectiveness of educative programmes and food-based interventions in increasing iodine intake and improving iodine status of pregnant women, as studies tend to focus on the success, harm and benefits of supplementation and salt fortification. Our systematic review of the literature from 1990 to 2016 identified a lack of intervention studies focusing on foods (rather than supplements and fortification) or educative programmes to increase iodine intake during pregnancy(Reference Bouga and Combet114). Of the three studies that met the inclusion criteria, one was a proposed study protocol(Reference Prieto, Torres and Frances115), another (LIMIT study, South Australia) was an intervention in overweight and obese women, at 10–12 weeks of gestation without specific focus on iodine(Reference Dodd, Cramp and Sui116), and the third was a RCT (Tehran, Iran) of pregnant women, between the 4th and 18th weeks of pregnancy(Reference Amiri, Hamzavi-Zarghani and Nazeri117). The RCT, the only piece of evidence directly linked to iodine, concluded that the intervention (a 4-month educational programme using face-to-face educational sessions, a leaflet in the second and the third trimesters, as well as telephone) increased knowledge, attitude and practice, but not iodine status. Iodine status was however reported as a median UIC of the groups, measured from a single spot urine sample, and may not be the most appropriate tool to evaluate changes in status in this small group. RCT are urgently needed to examine the effectiveness of different approaches as well as the long-term health, neurocognitive and economic effects on the population. Including food guidance as a dimension of any future intervention is a vital step before the implementation of policy and public health campaigns, which would also be socially and politically acceptable. The UK offers a great opportunity for further research, as it is an ideal terrain for interventions, lacking prophylaxis such as salt fortification and supplementation.
ID has been described as ‘the low-hanging fruit of public health’ in the UK(118). The challenge could be tackled through a range of strategies, including policy implementation (salt and staple foods iodisation, supplementation); educational campaigns for increased awareness in women and HCP; and development of comprehensive food-based guidance for the general population, pregnancy and lactation. However, none of those potential solutions is in place now in the UK, and the problem of insufficiency has been consistently overlooked. Recently, the Scientific Advisory Committee on Nutrition published an updated report on iodine, with no recommendations to revise the reference intake values(119), indicating that the existing evidence might not be sufficient for a policy revision. Governmental actions are required, and the UK should follow the example of other countries, such as the USA, Australia and New Zealand in policy for fortification and supplementation according to the WHO(16). The cases of cessation of water fluoridation (in Scotland) and absence of mandatory fortification for folate are two similar examples of potential missed opportunities to positively impact on population health, possibly through a more rigid policy-making framework compared with other Western nations. In less developed countries, focus on an increased household coverage with iodised salt, and addition of iodine to condiments, soyabean paste and sauce is driven by the Iodine Global Network/International Council for the Control of IDD strategy on global elimination of ID(Reference Codling120, 121). Co-existing deficiencies, such as iron, zinc and selenium, should also be taken into consideration(122), as they are important for thyroid function, improvement of the efficacy of iodine supplementation and prevention of myxedematous cretinism(Reference Zimmermann and Kohrle123). The WHO is targeting micronutrient deficiencies globally by proposing a balanced and diversified diet, micronutrient supplementation and fortification of foods (i.e. sugar, salt, maize, oil, rice, wheat) with micronutrients (folic acid, iron, calcium, vitamin A, B12, zinc)(122).
To address ID effectively, solutions should work synergistically. Changing dietary patterns is challenging, considering the unregulated commercial marketing of foods. The example of fruits and vegetables provides the evidence that dietary changes can happen, and interventions designed to increase a dietary component can be successful, although slow. Dietary change is however mostly effective in the subgroups of the populations, leaving the lower socioeconomic groups and those with the greatest need (e.g. low income, homeless, socially deprived, urban migrant groups) untargeted(Reference Pomerleau, Lock and Knai124, Reference Pomerleau, Lock and Knai125). This in itself calls for a multipronged approach to tackle ID, in the UK and globally, depending on the needs and iodine status of each population.
Acknowledgements
The authors would like to acknowledge Glasgow Children's Hospital Charity for their support.
Financial Support
M. B. is in receipt of a scholarship from Glasgow Children's Hospital Charity (grant number: YRSS-2014-05).
Conflict of Interest
None.
Authorship
M. B. gathered and critically appraised the literature and drafted the manuscript. E. C. and M. E. J. L. reviewed and contributed to the manuscript.







