Up to 90 % of the attributable causes of colon cancer may be environmental, mostly linked with diet and lifestyle(1, Reference Parkin, Bray, Ferlay and Pisani2). Although it was widely believed that high-fibre diets were protective against colorectal cancer, the reality appears to be more complex, and has been the subject of recent controversy. Ten years ago the suggestion that the data underpinning the ‘fibre hypothesis’ with respect to colon cancer was not as strong as had been implied attracted a lot of criticism(Reference Wasan and Goodlad3), especially the assertion that in some circumstances, colonic fermentation of carbohydrates could have adverse effects(Reference Goodlad4). Since then several large prospective and intervention studies have shown null effects(Reference Lawlor and Ness5, Reference Park, Hunter and Spiegelman6), with some showing evidence of increased risk(Reference Goodlad4, Reference Fuchs, Giovannucci, Colditz, Hunter, Stampfer, Rosner, Speitzer and Willett7, Reference Alberts, Martinez and Roe8). Other studies, most prominently a prospective investigation of a large European cohort, have found naturally fibre-rich diets to be associated with reduced risk of colon cancer(Reference Bingham, Day and Luben9). Despite the inconsistencies in the evidence relating to colon cancer, there is still general advice from health professionals to increase intake of natural fibre-rich foods because of their overall benefits, including the association with reduced incidence of CVD(Reference Pereira, O'Reilly and Augustsson10).
Dietary fibre as well as resistant carbohydrate preparations escape digestion in the small intestine and depending on the type can have a range of attributes in the colon including fermentation and bulking. The presence of nutrients in the intestinal lumen, with respect to either luminal nutrition or intestinal workload, has profound actions on the development and maintenance of the intestinal epithelium(Reference Goodlad, Plumb and Wright11, Reference Goodlad, Wilson, Lenton, Wright, Gregory and McCullagh12). Atrophy of the colon is observed with a resistant carbohydrate-free ‘elemental’ diet(Reference Sasaki, FitzGerald, Grant, Wright and Goodlad13), which can be reversed by resistant carbohydrates, but only in animals with an intestinal flora(Reference Goodlad, Lenton, Ghatei, Adrian, Bloom and Wright14). This effect is not seen in germ-free rodents(Reference Goodlad, Ratcliffe, Fordham and Wright15, Reference Pell, Johnson and Goodlad16), demonstrating that it is the products of fermentation (the SCFA), rather than bulk, that are trophic. Excessive and rapid fermentation in the colon has been linked to increased proliferation of the intestinal epithelium(Reference Goodlad, Ratcliffe, Fordham and Wright15, Reference Pell, Johnson and Goodlad16). As increased proliferation is generally considered to be a risk factor for carcinogenesis(Reference Preston-Martin, Pike, Ross, Jones and Henderson17), the desirability of consuming large amounts of rapidly fermented resistant carbohydrates needs to be questioned.
The present study compares the effects of a normal diet, a semi-synthetic diet and the semi-synthetic diet supplemented with resistant carbohydrate preparations of differing fermentabilities. The effects were investigated with normal mice and cancer-prone mice (adenomatous polyposis coli multiple intestinal neoplasia (Apc Min/+) mice). Intestinal cell renewal and crypt fission were measured in the small intestine and in the colon(Reference Wasan, Park, Liu, Mandir, Winnett, Sasieni, Bodmer, Goodlad and Wright18). Crypt fission is an alternate means of increasing intestinal tissue mass by creating new crypts and could be the main mechanism for the spread of mutant clones of cells in the gut(Reference Preston, Wong and Chan19, Reference Brittan and Wright20). The present study therefore addresses the effect of different fermentable substrates on cell proliferation and polyp formation as a model for investigating their potential influence on the development of gut cancer.
Methods
The effects of the diets on polyp number size and burden were measured in the Apc Min/+ mouse, which is generally considered to be a good pre-clinical model of gut cancer(Reference Corpet and Pierre21–Reference Goodlad and Alison23). The Apc Min/+ mouse is heterozygous at the Apc (adenomatous polyposis coli) locus (as occurs in familial adenomatous polyposis in man) and loss of the remaining wild-type allele leads to β-catenin accumulation and relocation to the nucleus, where it forms a complex with Tcf-4 leading to the transcription of tumour-promoting genes.
Apple pomace, the pulpy material remaining after apples have been pressed for juice extraction, was chosen as it is highly fermentable(Reference Swanson, Grieshop, Clapper, Shields, Belay, Merchen and Fahey24) and bran was chosen as it is lignified and less fermentable, and also because it was the same resistant carbohydrates as used in the Alberts intervention study(Reference Alberts, Martinez and Roe8). The NSP content of the two test materials were measured by the Englyst procedure(Reference Englyst, Quigley and Hudson25).
ApcMin/+ mice
Apc Min/+ heterozygote mice were originally obtained as a gift from Amy R. Moser (McArdle Laboratory for Cancer Research, University of Wisconsin, Madison, WI, USA)(Reference Moser, Mattes, Dove, Lindstrom, Haag and Gould26). Male mice were back-crossed to female C57BL/6J mice and the resultant embryos were transferred by aseptic hysterectomy to foster mothers in specific pathogen-free isolators. All breeding was subsequently by brother (C57BL/6J – Apc Min/+)–sister (C57BL/6J) mating. Genotyping was carried out by a PCR-based method, using three primers including an internal control for normal mouse DNA. On average, half the mice born in a litter will be Apc Min/+ and half will be wild type. All procedures were approved by the Cancer Research UK Animal Ethics Committees and covered by the appropriate licences under the Home Office Animal Procedures Act, 1986.
Study design
Four groups of fifteen female Apc Min/+ and fifteen female wild-type littermate mice were put on the following powdered diets prepared by Special Diets Services (Witham, Essex, UK): Group 1, chow diet based a standard mouse maintenance diet (RM1); Group 2, semi-synthetic diet; Group 3, semi-synthetic diet +20 % wheat bran; Group 4, semi-synthetic diet +20 % apple pomace. The bran was supplied by Trouw Nutrition (Witham, Essex, UK) and the dehydrated apple pomace (from fresh apples, variety ‘Rome Beauty’) was from Kanegrade Ltd (Stevenage, UK).
Autopsy and analysis
After 8 weeks, mice were injected with 1 mg vincristine/kg (to arrest cells as they enter metaphase) and killed 2 h later(Reference Goodlad and Celis27, Reference Alferez and Goodlad28). The small intestines and colons were isolated, rinsed and weighed. The small bowel was divided into three equal sections (proximal, middle, distal), dissected longitudinally using a recently described gut-cutting device(Reference Rudling, Kitau, Hassan, Mandir and Goodlad29) and spread on to filter paper. The entire colon was also dissected and spread on to filter paper. These gut preparations were then fixed in Carnoy's fixative for 3 h and then transferred to 70 % ethanol. The tissues were assessed later under a stereomicroscope ( × 20 magnification) for polyp number and diameter, which was measured using digital callipers. Polyp volume was derived from polyp diameter, assuming a hemispherical shape in the small bowel and a spherical shape in the colon. Tumour burden was calculated as the product of polyp number and polyp volume(Reference Bashir, FitzGerald and Goodlad30).
Assessment of proliferation and fission throughout the gut was performed using the ‘crypt microdissection’ method. This method is up to six times faster than scoring histological sections and avoids several problems associated with quantifying histological sections(Reference Alferez and Goodlad28).
Representative samples of tissue from the proximal, middle and distal small intestine and colon (taken from positions 10, 50 and 90 % of the total length of the small bowel or colon) were hydrated, hydrolysed and stained with the Feulgen reaction. The mucosal crypts were gently teased apart under a dissection microscope and the numbers of metaphases per crypt (mean of twenty crypts) and crypt fission events per 200 crypts were then determined. All samples were counted in a ‘blinded’ fashion.
Statistics
Results are presented as the means and their standard errors. Weight and proliferative data were tested by two-way ANOVA: the wild-type and Apc Min/+ mice were compared so that effects of diet (v. the semi-synthetic) and effects of Apc Min/+ could be revealed and also if there was an interaction between these two factors. Polyp data were tested by one-way ANOVA, and if an effect of treatment was seen, Dunnett's post hoc analysis was performed using the semi-synthetic diet as the reference comparison. Minitab Statistical Software (release 10.5 Xtra Minitab, Coventry, UK) was used.
Results
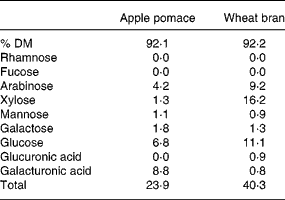
The NSP sugar content of the two preparations is shown in Table 1. The apple pomace contained 23·9 g NSP/100 g mainly as galacturonic acids, arabinose and galactose from easily fermentable pectin while the bran contained 40·3 % NSP mainly in the form of lignified less fermentable arabinoxylan and cellulose.
Table 1 NSP constituent sugars (g/100 g)
There were no differences in body weight between the different groups. The spleens, which are a useful surrogate marker of tumour load, were significantly heavier in the Apc Min/+ mice when compared to their wild-type littermates, except in the case of the bran-fed group where this was not seen, as reflected by significance effect of Apc Min/+ status, diet and interaction (Fig. 1).

Fig. 1 The effects of the various treatments on tissue wet weight (expressed as a percentage of body weight) for spleen (A), small intestine (D) and colon (E), and on the small intestinal (B) and colonic length (C). Min, multiple intestinal neoplasia (Apc Min/+) mice; WT, wild-type mice. The results of two-way ANOVA between the semi-synthetic (SS; control) diet and the various dietary modifications are indicated. These analyses test for effects of diet, of Apc Min/+ status and for interaction effects (int) between Min status and diet.
The small intestines were 10 % shorter and were 20 % lighter in the semi-synthetic groups compared to the chow (P < 0·001) and showed a modest weight increase of 7 % in the apple pomace-fed group (P < 0·02). There was also a small effect of Apc Min/+ status with the small intestines all heavier in the Apc Min/+ mice (11, 10, 4 and 4 % for the chow semi-synthetic and fibre diets, respectively, P < 0·04–0·001).
The colons were 15 % shorter and 37 % lighter in the semi-synthetic-fed mice when compared to the chow-fed mice, and were also slightly heavier in the Apc Min/+ mice. The bran and apple pomace-supplemented diets increased the length and the weights of the colon by 7 and 21 %, respectively (P < 0·015 and P < 0·001).
The effects of the various treatments on cell proliferation and crypt fission are shown in Fig. 2. Little difference in proliferation between groups was seen in the proximal small bowel, however, in the distal small intestine and the colon there were marked effects of both diet and Apc Min/+ status, with the Apc Min/+ mice having significantly greater metaphase counts compared to the wild-type mice (P < 0·02–0·001). Proliferation was reduced in the semi-synthetic-fed mice compared to the chow-fed mice (P < 0·02–0·001). Bran increased proliferation in the distal small intestine and in the colon (P < 0·01–0·001) while apple pomace only increased proliferation in the colon (P < 0·001).

Fig. 2 Cell proliferations, assessed by the 2 h accumulation of vincristine-arrested metaphases per crypt (A, C, E) and crypt fission indices (B, D, F) in the proximal and distal small intestine and mid colon. The sites were defined by their percentage length of the small intestine or colon: ((a, b), 10 % small intestine; (c, d), 90 % small intestine; (e, f), 50 % colon). Min, multiple intestinal neoplasia (Apc Min/+) mice; WT, wild-type mice. The results of two-way ANOVA between the semi-synthetic (SS; control) diet and the various dietary modifications are indicated. These analyses test for effects of diet, of Apc Min/+ status and for interaction effects (int) between Min status and diet.
Crypt fission in the proximal small intestine was increased in the Apc Min/+ mice of the chow and semi-synthetic and bran-fed (P < 0·02–0·03) and was slightly reduced in the bran-fed mice (P < 0·05), and appeared to be increased in the apple pomace group, which did not demonstrate an effect of Apc Min/+ status but did have a significant interaction effect (P < 0·05). A similar pattern was seen in the distal small intestine with significant effects of Apc Min/+ status being seen in the chow and bran-fed groups (P < 0·03–0·04) and significant effects of diet were seen in all the groups (P < 0·02–0·001). No effect of chow on crypt fission was seen in the colon, whereas both resistant carbohydrate-supplemented diets increased fission, especially in the Apc Min/+ groups. The effect of bran on the Apc Min/+ mice was particularly marked where there was a 6-fold increase (P < 0·001) and the apple pomace more than doubled fission (by 120 %, P < 0·03).
There were no polyps in the wild-type mice so Fig. 3 only shows the result from the Apc Min/+ mice. There was a significant increase in polyp number in the proximal third of the small intestine with bran, but no effect of bran was seen in the other sites. Polyp counts are lower in the proximal small intestine so that when the results for all the small intestine were pooled no effect of bran was observed. The apple pomace diet was associated with significantly increased polyp number throughout the small intestine (122, 236 and 92 % in the three sites, P < 0·001) and the total increase was 132 % (P < 0·001). No effects of the treatments on polyp number were seen in the colon.

Fig. 3 Polyp number in the three segments of the small intestine (A, proximal; B, middle; C, distal), the three segments together (D) and the colon (E). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of the control (semi-synthetic diet) group (post hoc comparison using Dunnett's test after one-way ANOVA): **P < 0·01, ***P < 0·001.
Fig. 4 shows the effects of the various diets on polyp diameter and there were no significant effects on diameter in the small intestine, although the diameter of the resistant carbohydrate-fed mice appeared to be smaller (Fig. 5). This thus reduced the significance of the increased polyp number so that the effect of apple pomace on the small bowel burden was reduced to an overall increase of 111 % (P < 0·05).

Fig. 4 Polyp diameter in the three segments of the small intestine (A, proximal; B, middle; C, distal), the three segments together (D) and the colon (E). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of the control (semi-synthetic diet) group (post hoc comparison using Dunnett's test after one-way ANOVA): *P < 0·05.

Fig. 5 Tumour burden in the three segments of the small intestine (A, proximal; B, middle; C, distal), the three segments together (D) and the colon (E). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those of the control (semi-synthetic diet) group (post hoc comparison using Dunnett's test after one-way ANOVA): *P < 0·05, ***P < 0·001.
Bran and apple pomace both significantly increased polyp diameter in the colon (by 60 and 40 %, P < 0·05) and when the product of number and diameter (burden) was calculated it can be seen that both types of resistant carbohydrate significantly increased polyp burden in the colon by 243 and 150 %, respectively (P < 0·05).
Discussion
The results of the present study have allowed us to demonstrate several effects of diet, Apc Min/+ status and their interrelationships. The Apc Min/+ mice showed significantly increased cell proliferation and crypt fission when compared to their wild-type littermates. Although the effects of the various diets were more pronounced in the Apc Min/+ mice, most were still seen in the wild-type which would suggest that the effects reported are not restricted to carriers of germ-line mutations in Apc.
There were no differences in polyp count or diameter between the chow and the semi-synthetic-fed mice. This is surprising as the intestines of the semi-synthetic fed mice were significantly shorter and lighter and had lower distal proliferative count. The semi-synthetic-fed mice also had lower fission in the distal small intestine. The present results are compatible with a reduced ‘luminal nutrition’ or ‘intestinal workload’ in the distal intestine and colon of the semi-synthetic-fed mice resulting in reduced proliferation rates, which has also been observed in rodents fed resistant carbohydrate-free elemental diets(Reference Sasaki, FitzGerald, Grant, Wright and Goodlad13).
The resolution of colonic events in the Apc Min/+ mouse is often rather limited, as there are usually very few polyps in the colon, nonetheless, both types of resistant carbohydrate were associated with significant increases in polyp diameter and this was particularly prominent in the bran-fed mice, which also had markedly increased fission indices in the colon. These mice also had increased proliferation, but so did the chow-fed group and there was no difference in the polyp diameters between the chow and the semi-synthetic group. The conclusion to be drawn is that increased fission in the colon is associated with increased polyp diameter, which then leads to increased polyp burden.
Bran and apple pomace supplementation both increased the number of polyps in the proximal small intestine, and while there are fewer polyps in this part of the small intestine, it may be more responsive to altered diet and growth factor signalling(Reference Bashir, FitzGerald and Goodlad30, Reference Bashir, Fitzgerald, Berlanga-Acosta, Playford and Goodlad31). Only the apple pomace was associated with an increased polyp number for the whole small intestine. For bran, no effect was seen in the middle or distal small bowel and it is of interest that the weight of the spleen was not increased in these mice when compared to their wild-type group. Spleen weight can be a useful indicator of tumour burden in the Apc Min/+ mouse(Reference Bashir, FitzGerald and Goodlad30, Reference Orner, Dashwood, Blum, Diaz, Li, Al-Fageeh, Tebbutt, Heath, Ernst and Dashwood32) and may be an indication of intestinal blood loss. The lower spleen weight in this group would thus suggest a protective role of the bran in the distal small intestine and although not significant there did appear to be a reduced polyp count, diameter and burden. The proliferative fission responses of this part of the gut were greater in the bran-fed group and fission was also greater which would indicate that it is only in the colon that increased fission leads to a larger polyp diameter. The role of crypt fission is still unfolding, but it has been proposed to be the main mechanism for the spread of mutant clones of cells in the gut(Reference Preston, Wong and Chan19, Reference Brittan and Wright20) and the present results in the colon are compatible with this.
There are many reports on the action of dietary fibre and other resistant carbohydrates on intestinal physiology with most studies showing that increased fermentation leads to increased cell proliferation, which is compatible with the concepts of luminal nutrition or intestinal workload, where the release of SCFA stimulates cell growth. These effects have been questioned, but mainly by those using in vitro models, which has led to the concept of the so-called ‘butyrate paradox’(Reference Lupton33, Reference Goodlad34). While in vitro models are very useful for studying molecular mechanisms, they may not be appropriate for the study of dietary agents. The gut is a complex multilayered defence system and has many mechanisms to protect its cells from extracellular chemicals, whereas in vitro the enterocytes are immersed in them. The results of the present study indicate that crypt fission is an important mechanism for increasing polyp size and they stress the crucial importance of in vivo studies.
Other groups have shown increased polyp number in Apc Min/+ mice with rapidly fermentable resistant carbohydrates and some now even use pectin-fed Apc Min/+ mice as a model of increased tumour load(Reference Pajari, Rajakangas, Paivarinta, Kosma, Rafter and Mutanen35, Reference Rajakangas, Pajari, Misikangas and Mutanen36); the same group also found fewer polyps in the distal small intestine of Apc Min/+ mice fed rye bran(Reference Mutanen, Pajari and Oikarinen37). Some of the earlier experiments in laboratory animals, using chemical induction of colon cancer, generally showed a protective effect with supplements of poorly fermentable resistant carbohydrates such as wheat bran or cellulose, while more rapidly fermentable resistant carbohydrate supplements including pectin, oat bran, undegraded carageenan, agar, psyllium, guar gum and alfalfa enhanced tumour development(Reference Jacobs38). These earlier findings are a matter of some concern as some rapidly fermentable resistant carbohydrates are now being promoted as ‘prebiotics’ due to their ability to alter the colonic flora in what is presumed to be a beneficial manner(Reference Cummings, Macfarlane and Englyst39, Reference Langlands, Hopkins, Coleman and Cummings40).
These earlier results suggest that the less fermentable brans may be better, nevertheless it should be remembered that more polyps recurred in women who had had one or more colorectal adenomas removed when given (less fermentable) wheat bran supplements for 3 years(Reference Alberts, Martinez and Roe8). A similar increase in polyp recurrence was also seen in both sexes of similar patients given the more fermentable resistant carbohydrate preparation, ispaghula(Reference Bonithon-Kopp, Kronborg, Giacosa, Räth and Faivre41).
The present study has focused on the effects of resistant carbohydrates on proliferation and crypt fission, but there are many other possible mechanisms by which resistant carbohydrates are likely to influence colon tumourigenesis, including several mechanical effects, such as bulking. Depending on the type, different resistant carbohydrates may also soften the stool, reduce intestinal transit, damp glycaemic response, bind carcinogens, bile acids, cholesterol and other potential toxins (but also essential nutrients), and can induce xenobiotic metabolising enzymes. Fermentation of resistant carbohydrates profoundly alters the colonic milieu and the release of SCFA will lower the pH, alter the flora and increase bacterial mass (and hence stool output). This acidification of the colon increases absorption of ferrous iron (the main form in supplements) and there is evidence that ferrous iron was positively associated with distal colon cancer among women who consumed more resistant carbohydrate(Reference Lee, Jacobs and Folsom42). Viscous resistant carbohydrates may also have independent actions on mucosal proliferation(Reference Gee, Lee Finglas, Wortley and Johnson43). There is also the question of whether SCFA and, in particular, butyrate, are ‘the preferred fuel’ for the colonocyte(Reference Roediger44) or whether it is the colonocyte's role to quickly remove these ‘toxic’ chemicals(Reference Goodlad4).
The inclusion of rapidly fermentable resistant carbohydrate substrates may perturb the colon; it has been proposed that rapid fermentation could lead to a ‘feast or famine’ scenario where in the famine the microorganisms must induce enzymes to ferment dying or dead microbes and the colonic epithelial mucosa and mucins. This proteolytic fermentation will generate ammonia and carcinogens, which could increase the probability of precancerous lesions and polyps developing(Reference McBurney, Van Soest and Jeraci45).
While both resistant carbohydrate preparations investigated increased cell proliferation, crypt fission and polyps, the effects varied depending on the location in the gut. Although the apple pomace represents a more fermentable substrate, a larger total amount of resistant carbohydrate was provided by the less fermentable bran in the present study, so that the overall fermentation occurring with the two diets was perhaps similar. It seems likely that both the amount and fermentability of resistant carbohydrates have an impact on the gut epithelium function.
The dietary fibre story is still unfolding, and different systems may respond differently, as a recent cohort study has indicated that dietary fibre can prevent breast cancer, but only in pre-menopausal women(Reference Cade, Burley and Greenwood46). Such large-scale intervention studies may eventually lead to a resolution of the role in colorectal cancer, but it seems that they are very susceptible to the actions of covariates. For example, a large meta-analysis initially showed that dietary fibre could be protective, but further analyses accounting for other dietary risk factors removed the association(Reference Park, Hunter and Spiegelman6). These methods were then used on the European Prospective Investigation into Cancer and Nutrition (EPIC) data and the 40 % reduction in risk associated with fibre(Reference Bingham, Day and Luben9) was removed(Reference Michels, Fuchs, Giovannucci, Colditz, Hunter, Stampfer and Willett47), although this has been recently challenged(Reference Bingham48).
Nevertheless, the general advice from health professionals to consume a natural high-fibre diet of fruit, vegetables and whole-grain products(Reference Jacobs, Andersen and Blomhoff49) is fully supportable, as there is convincing evidence that such diets are beneficial with respect to obesity, CVD, diabetes and some types of cancer(Reference Pereira, O'Reilly and Augustsson10, Reference Key, Allen, Spencer and Travis50, 51). In addition, natural fibre-rich diets, for which dietary fibre defined as ‘intrinsic plant cell wall polysaccharides’ is a good marker(Reference Englyst, Liu and Englyst52), are likely to contain co-passengers, such as the many different phytochemicals, which have been shown to exert effects on cell proliferation throughout the alimentary tract(Reference Johnson53).
The present study supports the hypothesis that fermentation of large amounts of resistant carbohydrates by gut bacteria may have potentially detrimental effects on colonic health. There is little evidence to suggest that the resistant carbohydrates in the amounts present in a natural fibre-rich diet represents a cancer risk. However, there is potential for concern if easily fermentable resistant carbohydrate preparations are consumed in large amounts and therefore these types of functional ingredients, including resistant oligosaccharides and resistant starch, should be researched for both short- and longer-term effects and, if shown beneficial to health, promoted individually.
Acknowledgements
We would like to thank all the staff at the BSU Clare Hall for their help and Ian Rosewell for the genotyping. H. E. is director and shareholder in Englyst Carbohydrates Ltd, which is a small research-oriented company focusing on dietary carbohydrates and health. R. A. G. has no conflicts of interest. Funding was provided by CRUK. N. M. was responsible for scoring the tissue and the preliminary analysis of the data. R. A. G. designed and organised the experiment, analysed the data and prepared the manuscript. H. E. advised on and analysed the resistant carbohydrates and significantly contributed to the introduction and discussion.










