Nutrition has the greatest effect on bone growth, as it influences the accumulation of minerals and therefore affects the intensity of bone formation and resorption processes in bone turnover. Dietary intake of energy, protein and mineral components (e.g. Ca, P) is known to be critical for attaining and maintaining a healthy skeletal reserve( Reference Gutzwiller, Hess and Adam 1 – Reference Skiba, Sobol and Raj 4 ). It has been known for decades that a diet rich in SFA can adversely affect bone mineralisation in growing rats( Reference Zernicke, Salem and Barnard 5 ) and quails( Reference Liu, Veit and Wilson 6 ). Recently, PUFA, particularly EPA (C20 : 5n-3), docosapentaenoic (DPA, C22 : 5n-3) and DHA (C22 : 6n-3), have drawn interest as natural substances that can affect bone metabolism at the cellular level( Reference Lau, Cohen and Ward 7 , Reference Parks 8 ). Supplementation of the diet with EPA and DHA (from fish oil) improved bone health in rodents( Reference Sun, Krishnan and Zaman 9 – Reference Fallon, Nazarian and Nehra 14 ), quails( Reference Liu, Veit and Wilson 6 , Reference Liu, Veit and Denbow 15 ) and piglets( Reference Weiler and Fitzpatrick-Wong 16 , Reference Mollard, Kovacs and Fitzpatrick-Wong 17 ). Parks( Reference Parks 8 ) suggested that both long-chain n-3 and n-6 PUFA have beneficial effects on bone modelling in children. However, the results of a study carried out by Judex et al. ( Reference Judex, Wohl and Wolff 18 ) demonstrated that dietary EPA and DHA (from fish oil) supplementation adversely affects cortical bone morphology and biomechanics in growing rabbits.
In North America and Europe, the majority of dietary intake of n-3 PUFA is derived from rapeseed and linseed oils (rich in α-linolenic acid (ALA, C18 : 3n-3)). A review of the available literature identified only a few studies that have investigated the effect of feeding animals a diet rich in ALA on bone health. However, these studies were carried out mainly on rodents( Reference Lau, Fajardo and McMeekin 11 , Reference Weiler, Kovacs and Nitschmann 19 ) and poultry( Reference Baird, Eggett and Fullmer 20 ) and the results were inconclusive. The studies were limited mainly to bone mineral density and maximum bone strength, whereas the health properties of bone are represented by a greater number of parameters (including geometry, densitometry and biomechanical indicators).
A review of the current literature( Reference Ratnayake and Galli 21 , Reference Kalish, Fallon and Puder 22 ) revealed that there are differences between the type and quantity of dietary n-3 PUFA on bone quality and structure. However, there was no simultaneous comparison of the effect of diets rich in ALA v. those rich in EPA and DHA on bone properties. Moreover, dietary n-6:n-3 PUFA ratio seems to have a great impact on bone parameters; however, this issue has only been studied in quails( Reference Liu, Veit and Wilson 6 ) and rodents( Reference Bhattacharya, Rahman and Sun 10 , Reference Judex, Wohl and Wolff 18 ). We consider that all mentioned above issues are essential for the prevention of common bone diseases in humans, such as osteopenia and osteoporosis. Therefore, the present study had two aims. Our first aim was to determine the effect of the dietary source of fats (differing in n-3 PUFA content and n-6:n-3 PUFA ratio) on morphometry, densitometry, geometry and biomechanical parameters of the femur in growing pigs. Our second aim was to define the relationships between both dietary and empty body n-3 PUFA content and n-6:n-3 PUFA ratio and bone properties.
Methods
The experimental procedures used throughout this study were performed in accordance with national/local ethical guidelines and were approved by the III Local Ethics Committee on Animal Experimentation at the Warsaw University of Life Sciences – SGGW, Poland.
Animals, housing and diets
The experiment was carried out on thirty-two gilts (Polish Large White×Danish Landrace). At 115 d of age (approximately 60 kg body weight), the pigs were divided into four groups (n 8 per group) and were fed either a control diet (not supplemented with any source of fat, group C) or a diet supplemented with linseed oil (rich in ALA, group L), fish oil (rich in EPA and DHA, group F) and beef tallow (rich in SFA, group T) for the next 50 d (until the pigs were 165 d of age, approximately 105 kg body weight). In experimental diets the amount of energy added as fat accounted for 10 % of metabolisable energy. Diets were iso-energetic (average 13·55 MJ metabolisable energy/kg) and iso-lysinic (average 8·20 g of standardised ileal digestible lysine/kg) but differed in their n-3 PUFA contents (0·63–18·52 g/kg) and n-6:n-3 PUFA ratios (0·91–14·51). The content of metabolisable energy, standardised ileal digestible amino acids and total P in the diets were calculated as detailed by nutrient requirements of swine( 23 ). Diets were supplemented with a mineral–vitamin mixture and crystalline amino acids, according to nutrient requirements of swine guidelines( 23 ). The composition, nutritive value and energy contents of each of the diets are presented in Table 1. The dietary fatty acid contents are presented in Table 2.
Table 1 Ingredients, chemical composition and nutritive value of diets
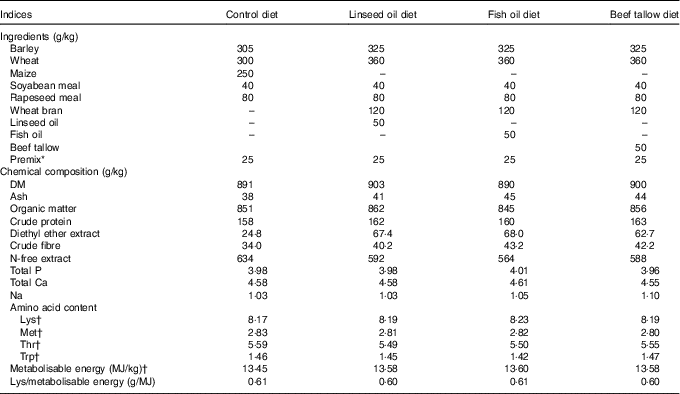
* Addition of 2·5 % premix (A – in control diet, B – in experimental diets) introduce to 1 kg of diet: vitamin A 450 μg, vitamin D3 7·5 μg; Fe 60 mg, Zn 50 mg, Cu 30 mg, Mn 30 mg, I 0·30 mg, Se 0·20 mg, vitamin E 40 mg (premix A) or 150 mg (premix B), vitamin K3 2·0 mg, vitamin B1 2·0 mg, vitamin B2 2·5 mg, vitamin B6 2·0 mg, vitamin B12 0·02 mg, biotin 0·11 mg, folic acid 0·6 mg, nicotinic acid 15 mg, calcium-d pantothenate 10 mg, choline chloride 500 mg; Ca 2·8 g, P 0·07 g and essential amino acids: lysine 2·63 g, methionine 0·68 g, threonine 0·98 g.
† Calculated according to nutrient requirements of swine( Reference Weiler, Kovacs and Nitschmann 19 ).
Table 2 Fatty acids content in the diets (g/kg)
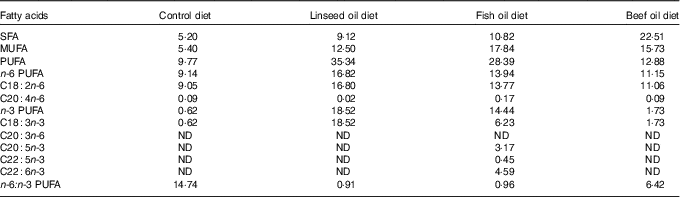
ND, value below 0·01 was classified as not determined.
The animals were kept individually in pens (3·3 m2) equipped with nipple drinkers, on a concrete floor without straw. Pigs had olfactory, auditory, visual and physical contact with each other. Dry, granulated feed was offered at a dose of 85 % ad libitum twice daily (at 08.00 and 14.30 hours). Restricted feeding allowed the full control of fatty acid intake. The environmental conditions in the piggery were as follows: air temperature (18–20°C), relative humidity (60–70 %) and air flow (0·2–0·4 m/s) regulated by a Fancom ventilation system (model ISM0.12; Fancom BV) and in accordance with the European Union law( 24 ).
Sample collection, measurements and calculations
At 165 d of age, the pigs were slaughtered. Before slaughter, pigs were electrically stunned (STZ 3 apparatus; P.P.H. MASTER Sp. J.), exsanguinated and scalded to remove hair. Blood was collected and weighed, the abdominal cavity was opened and the internal organs and the gastrointestinal tract were removed, emptied and weighed. The carcasses were weighed and then chilled at 4°C for 24 h, after which each right half-carcass was dissected into edible (meat and fat) and inedible (bone, skin) parts. Blood, internal organs and the gastrointestinal tract (offal) were autoclaved (130°C, 1·25 atmosphere pressure) for 8 h, as were all inedible parts. Following, a 500 g sample of each separated body parts was collected, homogenised and used for chemical analysis (protein, diethyl ether extract, ash) and fatty acid composition. Mass of edible and inedible parts was established in relation to the whole carcass. Empty body was calculated as the sum of edible, inedible and offal parts.
After exsanguination, the left femur was dissected. Following excision, the bones were cleaned of any remaining flesh, weighed and frozen (–20°C) for subsequent dual-energy X-ray absorptiometry scanning with a Norland XR-800™ densitometer scanner with a ‘Research Scan’ type (Norland, A Cooper Surgical Company). The dual-energy X-ray absorptiometry scans were obtained by standard procedure supplied by the manufacturer for scanning and analysis. A quality assurance test to verify the stability of the system calibration (control scans) was performed on a daily basis. A daily calibration procedure was performed using a QC Phantom and QA Calibration standard (Norland, A Cooper Surgical Company). Specimens for scanning were thawed at room temperature (23°C) for 12 h before use. During scanning, the femur was positioned horizontally with the femoral head facing upwards, and the condyles downwards, and then scanned from the distal to the proximal end. All the scans were performed in triplicate to avoid any rotation of the bone, as inconsistencies in their orientation can adversely affect the accuracy of test results. To ensure consistency, all scans were performed by the same operator. Bone mineral content and bone mineral density were recorded. After dual-energy X-ray absorptiometry scanning, the three-point bending test using a TA-HDi Texture Analyser (Stable Micro Systems Ltd) was applied to determine the biomechanical properties of the femur. The distance between supports of the bone was set at 40 % of the femur length and the measuring head loaded bone samples at the mid-shaft with a constant speed of 50 mm/min. The values of maximum bone strength and maximum bone elastic strength were determined. The geometrical properties of each femur were determined on the basis of the measurements of horizontal and vertical diameters (both external and internal) recorded after cutting the bone. The measurements were conducted using an electronic ruler.
The values of cortical wall thickness (mm), cross-section area (mm2) and cortical index (%) were determined using the following mathematical formulas:
where V is the vertical external diameter (mm), H is the horizontal external diameter (mm), v is the vertical internal diameter (mm) and h is the horizontal internal diameter (mm)( Reference Ferretti, Capozza and Mondelo 25 ).
Analysis of the diets
DM, N, ash, crude fibre, diethyl ether extract and simple sugar contents in the diets were determined as detailed by Horwitz & Latimer( 26 ) (procedures: 934.01, 984.13, 942.05, 978.10, 920.39 and 974.06, respectively). Protein content was estimated as N (g/kg)×6·25. To determine the fatty acid composition of the diets, 1·5 ml of potassium hydroxide was added to each dietary fat sample and the solution was heated at 75°C for 1 h. Next, methyl esters were prepared by esterification with thionyl chloride (4 % in methanol) and extraction with n-heptane. Fatty acid methyl esters were analysed using a GC-2010AF Shimadzu Gas Chromatograph (Shimadzu Europa GmbH) equipped with a flame ionisation detector. The derivatives were separated on a capillary column (BPX70, 60 m length, 0·25 mm internal diameter and 0·25 µm film thickness). The operating conditions were as follows: carrier gas, helium; split ratio, 1:100; injector and detector temperature, 260°C; the initial column temperature of 110°C was held for 5 min, then increased to 200°C at a rate of 3·5°C/min and was held for 2·5 min, then increased to 205°C at a rate of 0·3°C/min, then increased to 215°C at a rate of 1·5°C/min and was held for 3 min. Individual fatty acid peaks were identified by comparison with the Supelco 37 Component FAME Mix (SUPELCO) commercial standard. The total fatty acid content was calculated separately for each diet as being 90 % of the diethyl ether extract( 27 ).
Analysis of the empty body
The analysis of protein, diethyl ether extract and ash content in the empty body was calculated using the procedures described for the diets.
Lipids from samples of empty body were extracted with chloroform–methanol (2 : 1, v/v), homogenised and filtered. Then, 800 ml of filtrate was evaporated at 50°C (under N) and analysed according to the procedure described for dietary fatty acid analysis.
Statistical analysis
Statistical analyses were performed using Statgraphics Centurion (version 16.1.18, 2011) software (StatPoint Technologies Inc.). With an α level of 0·05, power established at 80 % and an effect size of 0·75, the required total sample size was 32 (i.e. n 8 per group). The hypothesised effect size of 0·75 was calculated from the descriptive statistics of a previous study( Reference Liu, Veit and Wilson 6 ). Post hoc calculations using the above-mentioned data indicated that the actual power achieved in this study was 83·1 %. Data are presented as means and standard deviations. The effect of diet on performance, fatty acids composition in the empty body and the femoral properties of pigs were analysed using a one-way ANOVA. When the F ratio was significant, Tukey’s honest significant difference post hoc analysis was performed. Statistical significance was set at P<0·05. A borderline significant trend was set at P<0·09.
The relationships between both dietary and empty body n-3 PUFA content and n-6:n-3 PUFA ratio and the geometric, biomechanic and densitometric properties of the femur were expressed as a linear regression model according to the following formula:
![]() $Y{\equals}a{\plus}{\beta}X$
$Y{\equals}a{\plus}{\beta}X$
where Y is the geometric or biomechanic or densitometric properties of the femur, a is the intercept, β is the slope ratio, X is the dietary n-3 PUFA content (expressed in g/kg diet) or n-6:n-3 PUFA ratio or empty body n-3 PUFA content (expressed in g/kg diet) or n-6:n-3 PUFA ratio.
Results
Fat and fatty acid contents in the diets
Diets L, F and T had similar amount of fat and fatty acids but higher than those of diet C (Table 2). The n-3 PUFA content was approximately 6·5 % (diet C), 13·5 % (diet T), 50·9 % (diet F) and 52·4 % (diet L) of the PUFA content of each diet. The ALA content was found to be the highest in diet L, much lower in diet F, lower in diet T and the lowest in diet C (18·52, 6·23, 1·73 and 0·62 g/kg, respectively). The EPA, DPA and DHA content were determined only for diet F (8·21 g/kg). The n-6:n-3 PUFA ratio took the following order 0·91 (in diet L), 0·96 (in diet F), 6·42 (in diet T) and 14·60 (in diet C).
Performance of animals
The average daily gain, daily feed intake (including both lysine and metabolisable energy), final protein, diethyl ether extract and ash contents measured in the empty body weight were not found to differ between the groups of pigs (Table 3).
Table 3 Performance of pigs during experimental period and final body composition (Mean values and standard deviations)
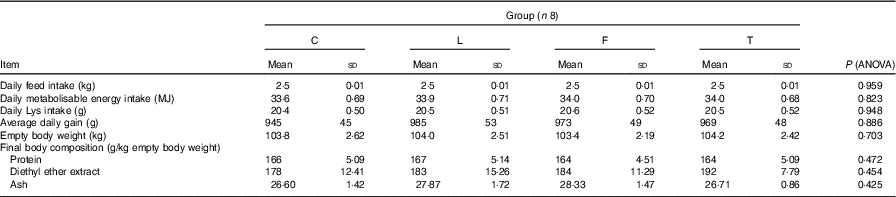
C, pigs fed a control diet; L, pigs fed a diet rich in α-linolenic acid (from linseed oil); F, pigs fed a diet rich in EPA and DHA (from fish oil); T, pigs fed a diet rich in SFA (from beef tallow).
Fatty acid content in the empty body
Dietary treatment affects the content of individual fatty acids in the empty body of pigs (Table 4). Content of SFA and MUFA did not differ among groups C, T and F; however, it was higher than in group L (P<0·05). The content of PUFA was the highest in group L, lower in group F and the lowest in groups C and T (P=0·010). Content of n-6 PUFA and LA took the following order: L>F>C>T (P=0·010). Empty body of T, C and F pigs had similar amount of arachidonic acid (AA, C20 : 4n-6), however, lower than in L pigs (P=0·007). Content of n-3 PUFA and ALA took the following order: L>F>T and C (P=0·010). Content of EPA, DPA and DHA was the highest in F pigs, lower in L pigs and the lowest in C and T pigs (P<0·010). The n-6:n-3 PUFA ratio in the empty body was similar in pigs of groups C and T, however higher compared with groups F and L (average 5·57, 1·71 and 1·20; P=0·006).
Table 4 Fatty acid content in the empty body of pigs at the end of the study (at 165 d of age) (Mean values and standard deviations)
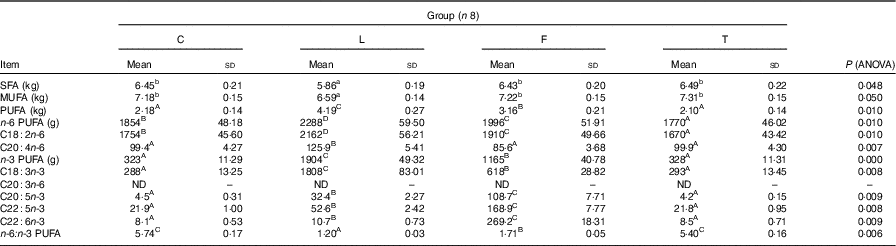
C, pigs fed a control diet; L, pigs fed a diet rich in α-linolenic acid (from linseed oil); F, pigs fed a diet rich in EPA and DHA (from fish oil); T, pigs fed a diet rich in SFA (from beef tallow); ND, value below 0·01 was classified as not determined.
A,B Mean values within a row with unlike superscript letters were significantly different (P<0·01).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Femur parameters
Dietary treatment was not found to have an effect on the mass and length of the femur (Table 5). The femurs of pigs in groups C and T had similar, though lower, cortical wall thickness (P=0·008), cross-sectional area (P=0·004), cortical index (P=0·016), bone mineral content (P=0·001), bone mineral density (P=0·005), maximum elastic strength (P=0·005) and maximum strength (P=0·003) than those of pigs in groups L and F.
Table 5 Femur morphometric, geometric, densitometric and biomechanical properties at the end of the study (at 165 d of age) (Mean values and standard deviations)
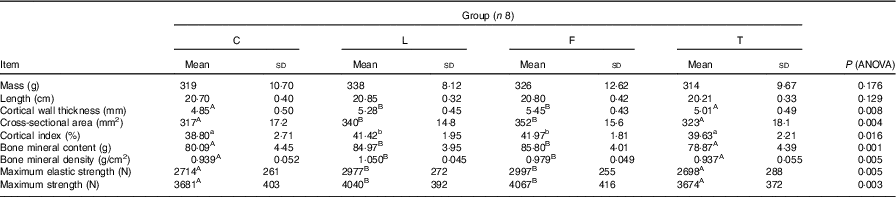
C, pigs fed a control diet; L, pigs fed a diet rich in α-linolenic acid (from linseed oil); F, pigs fed a diet rich in EPA and DHA (from fish oil); T, pigs fed a diet rich in SFA (from beef tallow).
A,B Mean values within a row with unlike superscript letters were significantly different (P<0·01).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
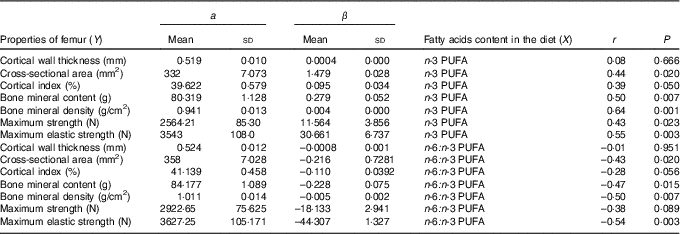
The relationships determined between dietary n-3 PUFA content and n-6:n-3 PUFA ratio and femur geometric, densitometric and biomechanical properties are shown in Table 6. Concerning relationships between dietary n-3 PUFA content and femur properties (except cortical wall thickness), a significant and positive correlations were found (r ranged from 0·39 to 0·64). In contrary, relationship between dietary n-6:n-3 PUFA ratio and cross-sectional area, bone mineral content, bone mineral density, maximum elastic strength of femur was significant and negative (r ranged from –0·43 to –0·54).
Table 6 Relationship (Y=a+βX) between femur properties and dietary n-3 PUFA content (g/kg) and n-6:n-3 PUFA ratio (n 32) (Mean values and standard deviations)
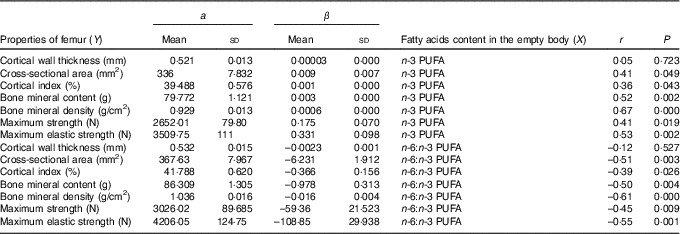
The relationships determined between the empty body fatty acid composition and femur geometric, densitometric and biomechanical properties are shown in Table 7. Relationships between n-3 PUFA content in the empty body and femur properties (except cortical wall thickness) were significant and positive (r ranged from 0·36 to 0·67). In contrary, relationships between n-6:n-3 PUFA ratio in the empty body and femur properties (except cortical wall thickness) were significant and negative (r ranged from –0·39 to –0·61).
Table 7 Relationship (Y=a+βX) between femur properties and empty body n-3 PUFA content (g/kg) and n-6:n-3 PUFA ratio (n 32) (Mean values and standard deviations)
Discussion
Limitations of the study
The presented results come from an experiment on the impact of n-3 PUFA on the health-promoting properties of pork. Therefore, in this article we did not deal with the effect of n-6 PUFA on bone properties. The femur was collected additionally as the bone most commonly used in human osteoporosis research. We focused on densitometry parameters as these factors are commonly measured in human medicine. Moreover, they have great impact on bone strength. This study, however, did not provide a complete picture of the influence of dietary n-3 PUFA on bone parameters, as we consider this effect only on the entire femur without taking into account bone microarchitecture. Undoubtedly, combination of adequate mineralisation and microarchitecture forms a strong and flexible bone highly resistant to fracture. However, differentiation of femur tissue into trabecular and cortical would allow to make our findings more precise. It is well known that trabecular bone is more metabolically active and undergoing more rapid remodelling than cortical bone, thus, the influence of dietary factors firstly occurs in trabecular tissue. Moreover, addition of fatty acids’ analyses from the animals’ serum undoubtedly improves the value of the manuscript, but unfortunately we have no such data. However, we have data concerning fatty acid content in the empty body. In our opinion, it is equivalent (or more precise) information as fatty acid content in the serum, because it reflects the total amount of fatty acids deposited by animal.
Furthermore, it was assumed that in the study on the influence of various types of fat sources on the investigated parameters, the pigs of control group should be fed diet not supplemented with any source of fat. As the study was carried out in the last period of pig fattening, feed ingredients of the diet C include the amount of energy that fully covered the requirements of pigs of this weight category. Therefore, additional source of energy (in fat form) was not required. Because SFA usually has a negative impact on bone properties( Reference Lorincz, Reimer and Boyd 28 , Reference Macri, Gonzales Chaves and Rodriguez 29 ), group of pigs fed diet T may be considered as a negative control group.
Animal performance and fatty acid contents in the empty body
As the energetic and nutritive values of pig diets as well as their feed intakes were equalised between the different groups of pigs, only their fatty acid intake differed, and this was dependent on the source of fat that was added to their diets. Therefore, the growth rate and fat and protein contents in the body did not differ between treatments. Previous studies carried out on pigs( Reference Kouba, Enser and Whittington 30 , Reference Olivares, Daza and Rey 31 ) and quails( Reference Liu, Veit and Wilson 6 ) showed similar response. In contrast, rats fed diets rich in n-6 PUFA (mainly linoleic acid (LA, C18 : 2n-6) from sunflower and soyabean oils) and broilers fed diets rich in n-3 PUFA (mainly ALA from linseed oil) had lower body fat accumulation compared with their counterparts that were fed diets rich in SFA (from beef tallow), as reported by Matsuo et al. ( Reference Matsuo, Takeuchi and Suzuki 32 ) and Crespo & Esteve-Garcia( Reference Crespo and Esteve-Garcia 33 ), Smink et al. ( Reference Smink, Gerrits and Hovenier 34 ), respectively. Crespo & Esteve-Garcia( Reference Crespo and Esteve-Garcia 33 ) suggest that a possible reason for the reduction in abdominal fat in broilers fed diets rich in ALA (from linseed oil) may be a result of higher lipid oxidation, as fats rich in n-3 PUFA are known to be more sensitive to oxidation. This effect has also been observed by other researchers( Reference Poureslami, Turchini and Raes 35 , Reference Skiba, Poławska and Sobol 36 ).
The results of the present study clearly showed that dietary fatty acids strongly influenced fatty acid content in the empty body, as pigs consumed diet rich in ALA (from linseed oil) and EPA and DHA (from fish oil) increased the amount of these fatty acids in their empty body compared with those animals fed diet rich in SFA (from beef tallow) and control diet. Other authors also demonstrate that dietary PUFA (both n-6 and n-3) modify fatty acid composition in rats’ bone tissue( Reference Li, Seifert and Lim 12 ) and in the liver and serum of piglets( Reference Weiler and Fitzpatrick-Wong 16 ). Our earlier study( Reference Skiba, Poławska and Sobol 36 ) showed that if pigs consumed a diet rich in ALA up to 82 % of consumed ALA can be directly deposited in the body. Moreover, such fed pigs assigned quantitatively higher amount of ALA to further bioconversion to longer-chain n-3 PUFA compared with animals fed the diets poorer in those fatty acids.
Effect of dietary source of fat on femur properties
The results of the present study indicate that the dietary source of fat, as a carrier of fatty acids, may affect bone health. Our study results confirm the findings of other studies( Reference Liu, Veit and Wilson 6 , Reference Lau, Cohen and Ward 7 , Reference Weiler and Fitzpatrick-Wong 16 , Reference Watkins, Shen and Allen 37 – Reference Weiss, Barrett-Connor and von Mühlen 39 ), indicating that a diet rich in n-3 PUFA may positively influence bone health. However, it is difficult to perform a direct comparison between presented and cited results due to species difference (rodents, poultry and piglets), examined bones and different amounts and sources of dietary fat used in the cited studies. Furthermore, the results of the studies carried out on rodents and poultry cannot be applied to human studies. However, our intention was to use pigs as an animal model for humans. Recent studies have demonstrated that, for humans, pigs are a better model than rodents and poultry owing to several similarities in anatomy, physiology, metabolism and pathology( Reference Pearce, Richards and Milz 40 – Reference Aigner, Renner and Kessler 42 ). Obviously, there are inter-species differences in the skeletal structure and load, however, our research interests focus on the effects of factors that affect the properties of pig and human bones in a similar manner. Therefore, in the present study, we decided to investigate the influence of the fatty acid content of diet and empty body on a range of femoral parameters (morphometric, geometric, densitometric and biomechanical) in growing pigs. These analyses will aid our understanding of the relationship between both dietary and empty body fatty acid composition and bone properties as well as the metabolic processes involved in this response.
The main finding of the present study carried out on a growing pig model is that consumption of a diet rich in ALA (from linseed oil) improved femur geometrical, biomechanical and densitometric properties to a similar extent as that observed following consumption of a diet rich in EPA and DHA (from fish oil).
Concerning the geometrical and biomechanical properties of the femur in the present study, we observed that consumption of diets rich in ALA (from linseed oil) and EPA and DHA (from fish oil) by pigs significantly improved these tested parameters compared with those of pigs fed a diet rich in SFA (from beef tallow) or a control diet. A review of the current literature did not reveal any information on the simultaneous comparison of the effect of diets rich in ALA v. EPA and DHA v. SFA on bone properties. Previous studies( Reference Liu, Veit and Wilson 6 , Reference Parks 8 – Reference Bhattacharya, Rahman and Sun 10 , Reference Baird, Eggett and Fullmer 20 ) indicate that longer-chain n-3 PUFA, particularly DHA, can have potentially favourable effects on bone health. Liu et al. ( Reference Liu, Veit and Wilson 6 ) showed that quails fed a diet rich in EPA and DHA (from fish oil) had higher cortical bone thickness and improved bone mineralisation and mechanical strength of tibia than those fed diets containing n-6 PUFA (from soyabean oil) and SFA (from poultry fat). Analogously, Reinwald et al. ( Reference Reinwald, Li and Moriguchi 43 ) found that rats fed diets deficient in n-3 PUFA had a significantly lower mechanical strength of the tibia compared with those fed diets with an adequate amount of n-3 PUFA. However, in this study the experimental factor did not affect the femur properties. It seems that the variation in response of animal on experimental treatment may depend on the type of bone being examined. The cited authors suggested that the tibia may be more sensitive than the femur to the action of dietary n-3 PUFA for bone modelling. Furthermore, Reinwald et al. ( Reference Reinwald, Li and Moriguchi 43 ) showed that dietary n-3 PUFA supplementation restored the tibia n-6:n-3 PUFA ratio and reversed compromised bone modelling in rats that had been fed a diet deficient in n-3 PUFA. In a study carried out on chickens, Baird et al. ( Reference Baird, Eggett and Fullmer 20 ) demonstrated that the cortical thickness of the tibia improved with the addition of increasing amounts of n-3 PUFA into the diet. However, in contrast to our results, bone strength was not found to be improved. Liu et al. ( Reference Liu, Veit and Wilson 6 ) suggest that the effect of dietary lipids on bone mineralisation is a cumulative process and that long-term dietary supplementation amplifies the effects of lipid turnover. The beneficial effects of n-3 PUFA on human bone health were also observed by Rousseau et al. ( Reference Rousseau, Kleppinger and Kenny 44 ). The cited authors observed a positive relation between the daily intake of n-3 PUFA and the femoral neck bone mineral density of elderly men and women (of 60 years of age or older). In contrast, the results of a study by Mollard et al. ( Reference Mollard, Kovacs and Fitzpatrick-Wong 17 ) carried out on piglets demonstrated that AA and DHA supplementation improved bone mineral content and density in lumbar spine but did not affect femur mineralisation. However, other researchers( Reference Baird, Eggett and Fullmer 20 ) were unable to demonstrate a positive effect of n-3 PUFA on animal bone parameters. A possible reason for the discrepancies between our findings and those of the cited authors may result from differences in bone growth, the type of bone used, difference between species and differences in the sex and age of the animals used in the studies. In general, the maximum rate of bone formation occurs during the earliest stage of animal growth (e.g. in pigs, this is during the first 12 weeks of life( Reference Heaney, Abrams and Dawson-Hughes 45 )). Thus, action of dietary treatment could take place at various times of bone/skeleton development. Difficulties in comparing the results of the studies indicated that further research is needed to determine the extent to which n-3 PUFA influences the metabolic activity of individual bones (long, flat and sesamoid) and mineral accretion at specific skeletal sites using animal model.
Concerning bone features measured using dual-energy X-ray absorptiometry method, we found that consumption of a diet rich in ALA (from linseed oil) improved femur mineral content and mineral density to a similar extent as that observed following the consumption of a diet rich in EPA and DHA (from fish oil). Fallon et al. ( Reference Fallon, Nazarian and Nehra 14 ) found a positive effect of diet rich in DHA on femur cortical mineral density and trabecular microstructure but not on trabecular mineral density. The results of the study carried out on growing rats by Li et al. ( Reference Li, Seifert and Lim 12 ) indicated that DHA accumulates in the osteoblast-rich and nerve-abundant periosteum of femur and appears to be a vital constituent of healthy modelling bone. Moreover, both cited authors found that consumption of a diet rich in DHA has a protective effect against trabecular bone loss, which is achieved among other by increase in the number of trabecular elements and subsequent strengthening of the trabecular network. The mechanisms by which dietary fat influences bone metabolism and function are not completely understood. Several mechanisms can be involved in the positive effect of diet rich in n-3 PUFA on bone health. Both LA and ALA are precursors of longer-chain n-6 and n-3 PUFA, respectively. These fatty acids compete for the enzyme Δ6-desaturase, which is necessary for de novo synthesis of longer-chain fatty acids( Reference Poureslami, Turchini and Raes 35 , Reference Turchini and Francis 46 ). Therefore, diets containing different amounts of these fatty acids introduce different amounts of substrates for n-6 and n-3 pathways. Previous studies( Reference Liu, Veit and Wilson 6 ) suggest that the action of ALA is achieved indirectly through its bioconversion, within the organism, into longer-chain n-3 PUFA. However, the efficiency of the bioconversion of ALA to longer-chain n-3 PUFA is dependent upon the expected end products( Reference Poureslami, Turchini and Raes 35 , Reference Skiba, Poławska and Sobol 36 , Reference Burdge and Calder 47 , Reference Kloareg, Noblet and Van Milgen 48 ). In humans( Reference Burdge and Calder 47 ), bioconversion of ALA to EPA (from 0·2 to 6 %) and DPA (from 0·13 to 6 %) is generally limited, and the synthesis of DHA is highly constrained (up to maximum 0·05 %). However, in pigs, bioconversion of ALA to longer-chain n-3 PUFA ranged from 3·7 to 6·3 %( Reference Skiba, Poławska and Sobol 36 ), some authors reported that even up to 13 %( Reference Kloareg, Noblet and Van Milgen 48 ). It seems that the second factor that affects the efficiency of this bioconversion appears to be the content of ALA in the diet( Reference Skiba, Poławska and Sobol 36 ). A study by Poureslami et al. ( Reference Poureslami, Turchini and Raes 35 ) demonstrated that dietary provision (abundance) of ALA promotes apparent elongation and desaturation activity on the n-3 PUFA pathway. In contrast, if the diet is supplemented with EPA and DHA (from fish oil), bioconversion (elongation and desaturation) of ALA is suppressed and a great amount of ALA is oxidised. In turn, the end products of these bioconversions act as substrates for other biologically active substances such as eicosanoids( Reference Liu, Veit and Denbow 15 , Reference Watkins, Shen and Allen 37 ). For example, the 3-series PG (PGE3) synthetised during bioconversion pathways of EPA and DHA have an anti-inflammatory effect which supports bone formation process( Reference Li, Seifert and Lim 12 ). In turn, 2-series PG (PGE2) synthesised during bioconversion of AA have a pro-inflammatory effect leading to the increase in the intensity of bone resorption process. Moreover, PGE2 is known to enhance receptor activator of NF-κB (RANK) expression in pre-osteoclasts, possibly leading to enhanced osteoclastogenesis and consequently bone resorption( Reference Liu, Kirschenbaum and Yao 49 ). Some researchers suggest that bioactive substances deliver from AA metabolism pathway (e.g. pro-inflammatory cytokines: IL-1, IL-6, TNF-α) involved in immune response that negatively affect bone growth and properties( Reference Das 50 ). On the other hand, various n-3 PUFA can reverse this trend in bone cells due to reduced osteoclastogenesis resulting from higher amount of resolvins in bone marrow and the associated reduction in gene expression connected with bone resorption process (e.g. TNF-α, RANK)( Reference Poulsen, Gotlinger and Serhan 51 ). Thus, it seems that EPA acts as a competitive inhibitor for production of bioactive substances derived from AA. It is well known that bioconversion of these fatty acids is supported by the same enzymes (e.g. cyclo-oxygenase and 5-lipoxygenase)( Reference Ratnayake and Galli 21 ). Moreover, some authors( Reference Classen, Coetzer and Steinmann 52 , Reference Heaney, Carey and Harkness 53 ) found that when feed was supplemented with n-3 PUFA, an increased intestinal Ca absorption and bone Ca deposition were observed. It could be attributed to dietary-induced increase in circulating PG which in turn has a positive effect on bone. An increased synthesis of PG acts on target bone cells as well as changes the membrane structure and fluidity. This change facilitates permeation of vitamin D, playing a crucial role in active transport of Ca across the cells membrane. Therefore, it can be concluded that if the content of EPA and DHA in the diet exceeds the content of AA, then less substrate is available for the synthesis of eicosanoids from AA. Thus, finally such fatty acid composition in the diet positively affects bone health.
The important role of n-3 PUFA on bone health also proves significant positive correlations between the femur geometric, densitometric and biomechanical parameters and dietary n-3 PUFA content determined in the present study. In addition, about the essential role of n-3 PUFA on bone health indicates significant negative correlations found between the same parameters and dietary n-6:n-3 PUFA ratio. Moreover, as the dietary fatty acid content in the feed offered to animals strongly affects their empty body content, it should not come as a surprise that a relationship for empty body n-3 PUFA content or n-6:n-3 PUFA ratio and investigated femur parameters happened to be similar, or even stronger, as in the case of dietary fatty acid composition. Similar correlations have been found previously by several authors in studies with rats( Reference Li, Seifert and Lim 12 , Reference Watkins, Li and Allen 38 ) and in elderly humans( Reference Weiss, Barrett-Connor and von Mühlen 39 , Reference Rousseau, Kleppinger and Kenny 44 ). However, Li et al. ( Reference Li, Seifert and Lim 12 ) correlated n-3 PUFA content and n-6:n-3 PUFA ratio in the marrow and periosteum of femur and tibia with properties of these bones. Studies in humans have been carried out by Weiss et al. ( Reference Weiss, Barrett-Connor and von Mühlen 39 ) and Rousseau et al. ( Reference Rousseau, Kleppinger and Kenny 44 ). These studies were based on self-administered FFQ alone. In the cited studies, dietary (or bone) n-3 PUFA content and n-6:n-3 PUFA ratio were found to correlate with bone mineralisation parameters (mineral content and density) as measured by X-ray absorptiometry. However, as the relationships of both dietary and empty body n-3 PUFA content and n-6:n-3 PUFA ratio with other bone parameters such as geometry and biomechanical parameters were not investigated previously, it is difficult to conduct closer discussion. Thus, evaluation of the relationships among dietary and empty body fatty acid composition and femur geometric, densitometric and biomechanical parameters are crucial for further understanding of the role of these factors in the formation of bone properties as well as the in the metabolic pathways involved in these processes.
In conclusion, along with increasing consumption of n-3 PUFA, femur parameters happened to be significantly improved. Contrary, if dietary n-6:n-3 PUFA ratio increases, the measure of bone parameters deteriorate strongly. Relationships for n-3 PUFA content and n-6:n-3 PUFA ratio in the empty body and investigated properties of the femur are similar, or even stronger, compared with the case of dietary fatty acid composition. Furthermore, linseed oil appeared to have a positive effect on bone parameters similar to fish oil.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. S., G. S. and S. R. conceived and designed the study, interpreted the data and drafted the manuscript. M. S. conducted the research and acquired the data and performed the statistical analysis.
The authors declare that there are no conflicts of interest.












