In ancient civilisations, cocoa (Theobroma cacao seed product) used to be consumed for its beneficial effects on health(Reference Hurst, Tarka and Powis1–Reference Keen3), but it changed from being consumed for medicinal purposes to being eaten as a confectionery in modern society. Nowadays, there is a resurgence of interest in the health properties of cocoa and its derivatives. Much scientific evidence has suggested that the beneficial effects of cocoa are associated with its flavonoids(Reference Rusconi and Conti4). Cocoa mainly contains flavan-3-ols such as ( − )-epicatechin and (+)-catechin (0·20–3·50 mg/g), and their polymers called proanthocyanidins (2·16–100 mg/g)(Reference Gu, House and Wu5, Reference Tomas-Barberan, Cienfuegos-Jovellanos and Marín6), which, unlike other products such as tea, apples, grapes or red wine, can have up to ten linked units of flavanol monomers(Reference Natsume, Osakabe and Yamagishi7). Large proanthocyanidins are less efficiently absorbed in the small intestine than shorter flavanols, and as a result they reach to the colon, where they are transformed by intestinal microbiota and absorbed through the intestinal barrier(Reference Monagas, Urpi-Sarda and Sánchez-Patán8). In consequence, the beneficial effect of these phytochemicals could be mainly attributed to metabolites derived from the microbial catabolism of proanthocyanidins(Reference Aura9, Reference Selma, Espín and Tomás-Barberán10). Large proanthocyanidins in the colon may have an important local function neutralising oxidants and carcinogenic compounds all along the gut(Reference Manach, Scalbert and Morand11). On the other hand, in addition to flavanols, quercetin and its derivatives, such as quercitrin, isoquecitrin, rutin, naringenin, luteolin and apigenin, are also present in cocoa in smaller quantities(Reference Sánchez-Rabaneda, Jáuregui and Casals12).
Despite some in vitro studies, the influence of cocoa on the immune system is still relatively unknown. Previously, we reported that a cocoa-enriched diet modifies lymphocyte composition and function in several lymphoid tissues in rats(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero14). Specifically, a 10 % cocoa diet given to young rats over 3 weeks increases the proportion of B-cells in the spleen(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13), mesenteric lymph nodes and also in Peyer's patches(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero14), whereas it down-regulates serum IgG, IgM and IgA production(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13). Moreover, specific antibody concentration against ovalbumin significantly decreased in immunised rats fed cocoa(Reference Pérez-Berezo, Ramiro-Puig and Pérez-Cano15). On the other hand, a cocoa-enriched diet reduces CD4+ T-helper (Th) lymphocyte proportion in the spleen and mesenteric lymph nodes but its IL-2 secretion, a cytokine with an autocrine effect producing Th proliferation, is not modified(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13).
Rheumatoid arthritis is an inflammatory autoimmune disease mainly mediated by Th cells(Reference Chen16). Adjuvant arthritis (AA) is an experimental model in rats of human rheumatoid arthritis that has been widely used for the screening of anti-inflammatory drugs(Reference Waksman17, Reference Holmdahl, Lorentzen and Lu18). The pathogenesis of the adjuvant arthritic process seems to be mainly related to CD4+ T-cells, because administration of monoclonal antibodies (mAb) against CD4 can prevent AA development(Reference Pelegrí, Morante and Castellote19) and also ameliorate established AA(Reference Pelegrí, Morante and Castellote20).
Inflammation is a complex biological response by vascular tissues to harmful stimuli to remove the injurious agent, as well as to initiate the tissue reparation process(Reference Medzhitov21). Studies performed in vitro have shown the anti-inflammatory properties of isolated flavonoids. In this sense, epicatechin and isoquercitrin decrease TNF-α and monocyte chemoattractant protein-1 production by lipopolysaccharide-stimulated macrophages(Reference Ramiro, Franch and Castellote22). Furthermore, quercetin inhibits cyclo-oxygenase pathways and PGE2 synthesis in the Chang liver cell line(Reference García-Mediavilla, Crespo and Collado23) and in human lymphocytes(Reference de Pascual-Teresa, Johnston and DuPont24). In addition, some studies have reasserted the anti-inflammatory capacity of isolated flavonoids in vivo. Subcutaneous or intravenous injections of catechin and epicatechin produce a significant reduction of a local acute inflammation induced in rats(Reference Matsuoka, Hasegawa and Okuda25).
The effect of cocoa on the inflammatory response is more complex than that of flavonoids because it contains a mix of diverse compounds. There are studies about the in vitro effect of cocoa on pro-inflammatory cytokine and chemokine release, generating controversial results. While some cocoa flavonoid fractions increase the production of pro-inflammatory cytokines in peripheral blood mononuclear cells(Reference Mao, van de Water and Keen26–Reference Jenny, Santer and Klein29); a complete cocoa extract decreases the TNF-α, monocyte chemoattractant protein-1 and nitric oxide production by macrophages(Reference Ramiro, Franch and Castellote22). In addition, peritoneal macrophages obtained from rats fed with cocoa for at least 1 week produce lower amounts of TNF-α, IL-6, NO and reactive oxygen species(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13, Reference Ramos-Romero, Ramiro-Puig and Pérez-Cano30–Reference Ramos-Romero, Pérez-Cano and Pérez-Berezo32). Moreover, in previous studies(Reference Ramos-Romero, Ramiro-Puig and Pérez-Cano30, Reference Castell, Franch, Ramos-Romero and Keller31), a cocoa suspension administered by the oral route for a week decreased the local hind-paw swelling induced by carrageenin and bradykinin in rats and reduced TNF-α concentration in inflammatory exudates. How cocoa metabolites down-regulate the inflammatory response remains to be established. Some in vitro studies have shown that flavonoids such as epicatechin, catechin, dimeric procyanidins and quercetin can modify the NF-κB pathway(Reference Mackenzie, Carrasquedo and Delfino33, Reference Comalada, Camuesco and Sierra34) involved in the synthesis of inflammatory products.
Because cocoa inhibits the release of some inflammatory mediators in vitro, reduces acute local inflammation in rodent models and decreases the Th-cell proportion in several lymphoid tissues in vivo, the aim of the present study was to determine the effects of two cocoa-enriched diets on AA. Specifically, we have taken into consideration not only clinical and biochemical inflammatory indices, but also antibody response and lymphocyte composition in two different compartments.
Materials and methods
Animals
A total of forty-five 9-week-old female Wistar rats were obtained from Harlan (Barcelona, Spain). Rats were housed three to four per cage in controlled temperature (20 ± 2°C) and humidity (55 %) conditions in a 12 h light–12 h dark cycle, with free access to food and water. Handling was done in the same time range to avoid the influence of biological rhythms. Studies were performed in accordance with the institutional guidelines for the care and use of laboratory animals established by the Ethical Committee for Animal Experimentation at the University of Barcelona and approved by the Catalonian Government.
Induction and assessment of adjuvant arthritis
To induce the arthritis process, rats were injected intradermally into the base of the tail with a suspension of 0·5 mg heat-killed Mycobacterium butyricum (Mb; Difco, Detroit, MI, USA) in 0·1 ml of liquid vaseline. AA was clinically assessed by means of hind-paw volume with a water plethysmometer (LI 7500; Letica, Barcelona, Spain). Left and right hind-paw volumes were measured just before AA induction (on day 0), daily during the second week post-induction, and every other day until the end of the study. All determinations were performed in a blinded manner. Articular inflammation was expressed as a percentage of increase in both hind-paw volumes with respect to their value on day 0. On day 14, animals were classified as arthritic if the increase in hind-paw volumes was higher than the volume increase media of healthy animals fed a standard diet (REF) plus two times its standard deviation.
Experimental design
The standard diet corresponded to the American Institute of Nutrition-93M formulation, which provides the nutrients required for optimal rat maintenance and was used as the reference diet (Table 1). Partially defatted Natural Forastero cocoa (Nutrexpa, Barcelona, Spain) was used to manufacture cocoa chows. This cocoa powder contained 22 % proteins, 16 % carbohydrates, 11 % lipids and 25·5 % fibre, and 21·2 mg of total phenols/g according to the Folin–Ciocalteu method. Cocoa diets (5 and 10 %) were prepared partially removing maize starch, soyabean oil, cellulose and casein from American Institute of Nutrition-93M standard starch and adding 50 or 100 g cocoa/kg of chow, respectively (Table 1). In consequence, the resulting chow had the same proportion of carbohydrates, lipids, proteins and total energy as the standard diet. Following the conversion of animal doses into human equivalent doses described by Reagan-Shaw et al. (Reference Reagan-Shaw, Nihal and Ahmad35), the 5 % cocoa-enriched diet was equivalent to 0·454 g cocoa/kg human per d (27·25 g cocoa for a 60 kg individual) and the 10 % cocoa-enriched diet was equivalent to 0·908 g cocoa/kg human per d (54·5 g cocoa for a 60 kg individual).
Table 1 Composition of the experimental diets (g/kg)*
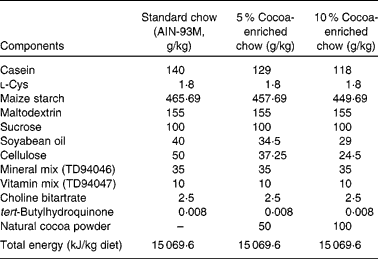
AIN, American Institute of Nutrition.
* The cocoa powder used contained 22 % proteins, 16 % carbohydrates, 11 % lipids and 25·5 % fibre, and 21·2 mg of total phenols/g according to the Folin–Ciocalteu method.
Animals were randomly distributed into four different experimental groups: REF (n 12); arthritic animals fed a standard diet (REF-AA; n 11); arthritic animals fed a 5 % cocoa-enriched diet (C5-AA; n 11); arthritic animals fed a 10 % cocoa-enriched diet (C10-AA; n 11). The REF and REF-AA groups were fed with the standard diet, the C5-AA group was fed with the 5 % cocoa-enriched diet and the C10-AA group with the 10 % of cocoa-enriched diet. The diets began 14 d before arthritis induction and lasted until the end of the study, a total of 6 weeks (Fig. 1).

Fig. 1 Diagram of the experimental design beginning 14 d before adjuvant arthritis (AA) induction until day 28 post-induction. ILN, inguinal lymph node.
At weeks 2 and 3 post-induction, the animals were anaesthetised by isoflurane inhalation in order to collect 100 μl of blood by tail vein puncture for serum anti-Mb antibody determination (Fig. 1). On day 28 post-induction, the animals were anaesthetised with ketamine (90 mg/kg; Merial, Lyon, France) and xylazine (10 mg/kg; Bayer HealthCare, Kiel, Germany) intraperitoneally and killed by total exsanguination by cardiac puncture. Blood and lymphoid tissues such as spleen, representative of the systemic immune tissues, and inguinal lymph nodes (ILN), that drain the knee joint synovia, one of the most affected tissues in AA, were obtained. An aliquot of each blood sample was used to automatically count the leucocytes by using a Coulter Counter JT haemocytometer (Hialeah, FL, USA) calibrated for rat blood. The differential white blood count was obtained by manual enumeration of May–Grünwald–Giemsa-stained blood cell smears.
Cell isolation from peripheral blood, lymph nodes and spleen
On the day of killing, blood was immediately treated with 10 g/l NH4Cl solution to lyse erythrocytes. After washing once with PBS containing 2 % fetal bovine serum (FBS; PAA, Pashing, Austria) and 0·1 % NaN3, peripheral blood cells were ready for immunofluorescence staining.
ILN and spleen were broken up by passing the tissues through a steel mesh (Cellector™; Bellco, Vertieb, Austria) with Roswell Park Memorial Institute-1640 media containing 10 % FBS (RPMI-FBS). Cells were then centrifuged (540 g, 10 min, 4°C) and resuspended in PBS. Then, lymphocytes from ILN were ready for immunofluorescence staining. The spleen cells underwent an erythrocyte lysis by adding distilled water for 5 s and restoring tonicity by adding PBS ten times. Then, cells were washed and resuspended with RPMI-FBS containing 100 000 U/l penicillin and 0·1 g/l streptomycin 2 mm-l-glutamine (Sigma Chemical Company, St Louis, MO, USA), and 0·05 mm-2-mercaptoethanol (Merck KGaA, Darmstadt, Germany) to be cultured. Number and viability were determined by acridine orange and ethidium bromide (Sigma) staining followed by fluorescence light microscopical analysis.
Immunofluorescence staining and flow cytometry analysis
Lymphocyte phenotype was determined just after cell isolation by double or triple staining, using fluorochrome-conjugated mAb followed by flow cytometry analysis. Mouse anti-rat mAb conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein or allophycocyanin used here included the following: anti-T-cell receptor (TCR) αβ (R73), anti-TCRγδ (V65), anti-NKR-P1A (10/78), anti-CD4 (OX-35), anti-CD25 (IL-2Rα chain, OX-39), anti-CD8α (OX-8), anti-CD45RA (OX-33), anti-CD81 (Eat2) (BD Biosciences, Erembodegem, Belgium) and anti-Foxp3 (FJK-16a; eBioscience, Frankfurt, Germany). Extracellular staining was performed in 5 × 105 cells by saturating concentrations of fluorochrome-mAb in PBS containing 1 % FBS and 0·1 % NaN3 (30 min, 4°C, in the dark). A negative control staining using an isotype-matched mAb was included for each sample. For intracellular staining, cells previously labelled with anti-CD4-phycoerythrin and anti-CD25-fluorescein isothiocyanate mAb were treated with a Foxp3 fixation/permeabilisation kit (eBioscience). Then, intracellular staining with anti-Foxp3-APC mAb was carried out in the same conditions as extracellular staining. All stained cells were fixed with 0·5 % p-formaldehyde and stored at 4°C in the dark. Analysis was performed using a Cytomics FC500-MPL cytometer (Beckman Coulter, Miami, FL, USA).
Lymphocyte subsets were defined in the cytometer as follows: Tαβ (TCRαβ+ NKR-P1A−), Tγδ (CD8+ TCRγδ+), B (CD45RA+), natural killer (NK; NKR-P1A+ TCR-αβ−), natural killer T (NKT; NKR-P1A+ TCRαβ+), Th (CD4+ TCRαβ+), T-cytotoxic (Tc; TCRαβ+ CD8α+ NKR-P1A−), Th2 (CD4+ TCRαβ+ CD81+), activated Th (Tact; CD4+ CD25+ Foxp3−) and regulatory Th (Treg; CD4+ CD25+ Foxp3+). Results are expressed as a percentage of positive cells in the lymphocyte population, selected previously according to the forward and side scatter characteristics of a cellular suspension that includes positive cells stained with anti-TCR, anti-NKR-P1A and anti-CD45RA. In some cases, results are presented as a percentage of positive cells in a specific lymphocyte subset (T, Th or NK cells).
Anti-Mycobacterium butyricum antibodies in serum
Anti-Mb antibody levels in sera were determined by using an indirect ELISA technique, as described previously(Reference Franch, Cassany and Castellote36). Briefly, polystyrene microELISA plates (Nunc Maxisorp, Wiesbaden, Germany) were incubated with a soluble protein fraction of Mb in PBS (3 μg/ml). After sample incubation, peroxidase-conjugated goat anti-rat Ig antibodies (BD Biosciences) were used. Since standards were not available, several dilutions of pooled sera from REF-AA animals were added to each plate. This pool was arbitrarily assigned 64 000 U/l anti-Mb antibodies.
Spleen cell culture and cytokines and PGE2 secretion
Splenocytes were cultured at 3 × 106 cells/ml in twenty-four-well plates. Cells were stimulated with Mb (10 μg/ml) for 24, 48 or 72 h or remained without a stimulus. IL-2 concentration was quantified in the 24 h supernatant using a rat ELISA set (BD Biosciences). Interferon γ (IFN-γ) concentration was determined in the 72 h supernatant with a Biosource ELISA set (Nivelles, Belgium). PGE2 concentration was determined in the 48 h supernatant by a competitive immunoassay kit from Cayman Chemicals (Ann Arbor, MI, USA) according to the manufacturers' recommendations. The time points to perform these analyses were established in a preliminary study by determining the maximal concentrations of these analytes in our culture conditions.
Statistics
The software package SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Levene's and Kolmogorov–Smirnov's tests were applied to assay variance equality and normal distribution of the studied groups, respectively. The one-way ANOVA followed by Scheffé's post hoc significance test was applied when the assumptions of normality and equal variance were met. In the opposite case, non-parametric tests (Kruskal–Wallis and Mann–Whitney U) were used to assay significance. Significant differences were accepted when P < 0·05.
Results
Effect of the cocoa diet on body weight and articular inflammation
At the beginning of the study, body weight was 165·2 (sem 1·1) g for all the studied groups. After 6 weeks of the diet, body weight was 231·8 (sem 3·6) g for the REF group, 200·2 (sem 5·4) g for the REF-AA group, 198·9 (sem 2·4) g for the C5-AA group and 192·9 (sem 4·0) g for the C10-AA group. Therefore, regardless of the diet, at day 28 post-induction, all AA animals presented similar body weight, which was lower than that of healthy animals (P < 0·05).
On day 14 post-induction, arthritis incidence was over 92 % for the reference arthritic group (REF-AA), similar to that found in both cocoa-fed groups (C5-AA and C10-AA). The time course of hind-paw volume increase after arthritis induction is summarised in Fig. 2(a). Paw volume reached a maximum increase of 130 % in the REF-AA group on day 21 post-induction. Animals from the C5-AA group displayed a lower paw volume increase than the reference group from day 16 to 28 post-induction, but differences were not statistically significant. On the last day of the study, the C5-AA volume increase was reduced by 32 % of that of the REF-AA group (Fig. 2(b)). Animals from the C10-AA group showed a paw oedema pattern similar to the REF-AA group, but on the last day of the study, the values were 28 % lower than those of the REF-AA (Fig. 2(b)).

Fig. 2 Effect of cocoa diets on the clinical evolution of adjuvant arthritis (AA) evaluated by hind-paw volume increase, measured by a water plethysmometer. (a) Time course of AA (expressed as a percentage of increase in both hind-paw volumes with respect to their value on day 0): ![]() , healthy animals fed a standard diet (REF);
, healthy animals fed a standard diet (REF); ![]() , arthritic animals fed a standard diet (REF-AA);
, arthritic animals fed a standard diet (REF-AA); ![]() , arthritic animals fed a 5 % cocoa-enriched diet (C5-AA);
, arthritic animals fed a 5 % cocoa-enriched diet (C5-AA); ![]() , arthritic animals fed a 10 % cocoa-enriched diet (C10-AA). (b) Percentage of hind-paw volume increase on the last day of the study. Values are means, with their standard errors represented by vertical bars (n 11–12). * Mean values were significantly different from those of the REF group (P < 0·05; ANOVA followed by Scheffé's test).
, arthritic animals fed a 10 % cocoa-enriched diet (C10-AA). (b) Percentage of hind-paw volume increase on the last day of the study. Values are means, with their standard errors represented by vertical bars (n 11–12). * Mean values were significantly different from those of the REF group (P < 0·05; ANOVA followed by Scheffé's test).
Effect of the cocoa diet on peripheral blood lymphocyte subsets in adjuvant arthritis rats
AA induced leucocytosis due to a neutrophil increase while the lymphocyte counts remained in the blood (Table 2). Alterations induced by AA were not modified by the cocoa diet.
Table 2 Effect of adjuvant arthritis and cocoa diets on blood leucocyte, lymphocyte and neutrophil counts
(Mean values with their standard errors)
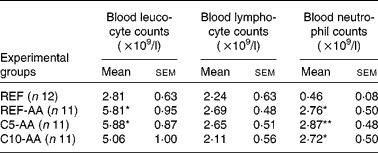
REF, healthy animals fed a standard diet; REF-AA, arthritic animals fed a standard diet; C5-AA, arthritic animals fed a 5 % cocoa-enriched diet; C10-AA, arthritic animals fed a 10 % cocoa-enriched diet.
Mean values were significantly different from those of the REF group (one-way ANOVA followed by Scheffé's test): *P < 0·05, **P < 0·001.
Percentages of blood Tαβ, Tγδ, B, and NK lymphocytes did not change significantly in the REF-AA group (Fig. 3(a)). In the case of arthritic rats, all groups showed lower blood NKT cell percentages than the reference healthy animals (REF). Moreover, the blood Tαβ cell percentage in animals fed a 10 % cocoa-enriched diet decreased with respect to the REF-AA group (P < 0·05).

Fig. 3 Lymphocyte subset composition in rat blood, determined by double or triple staining, using fluorochrome-conjugated monoclonal antibodies followed by flow cytometry analysis. (a) Tαβ, Tγδ, B, natural killer (NK) and natural killer T (NKT) lymphocyte percentages. (b) T-helper (Th) and T-cytotoxic (Tc) cell percentages in the T-cell population and Th:Tc ratio. (c) Th2, activated Th (Tact) and regulatory Th (Treg) percentages in the Th subset. (d) CD8+ and CD8− cell percentages in NK lymphocytes. Values are means, with their standard errors represented by vertical bars (n 9–12, with the exception of B-cells n 5–9). * Mean values were significantly different from those of healthy animals fed a standard diet (REF, ![]() ) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (
) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (![]() ); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
With regard to T-cell subsets (Fig. 3(b)), AA increased the Th-cell proportion (P < 0·05) and decreased the Tc-cell percentage (P < 0·05), resulting in a higher Th:Tc ratio in REF-AA animals compared with the REF group. Animals fed a 10 % cocoa-enriched diet avoided this Th:Tc imbalance caused by the arthritic process (P < 0·05 v. REF-AA). The Th:Tc ratio in rats from the 5 % cocoa diet group remained similar to that of REF animals, although values were not statistically different from the REF-AA group.
The proportion of Th2 cells was assessed by means of the presence of CD81 in Th cells. As shown in Fig. 3(c), the arthritic process did not affect the proportion of these cells, but both cocoa diets tended to reduce the Th2 proportion. Moreover, the AA process reduced the Treg (CD4+ CD25+ Foxp3+) cell proportion with respect to the REF group (P < 0·05; Fig. 3(c)) and, interestingly, the 10 % cocoa-enriched diet avoided this alteration. Tact cell proportion, determined as CD4+ CD25+ Foxp3− lymphocytes, was not affected by AA or the cocoa diets. NK cell subsets determined by CD8 phenotype (Fig. 3(d)) were not modified either by the AA process or cocoa intake.
Effect of the cocoa diet on inguinal lymph node lymphocyte subsets in adjuvant arthritis rats
The study of the main lymphocyte populations in ILN (Fig. 4(a)) revealed almost no changes induced by the arthritic process, as seen in the REF-AA group. There was only an increase in the low percentage of NKT cells (P < 0·05), which was also found to a lesser degree in the C5-AA and C10-AA groups. The cocoa diets decreased the B-cell population in AA rats in comparison with the REF group (P < 0·05; Fig. 4(a)), and there was a concomitant increase in Tαβ cells in the C5-AA group.

Fig. 4 Lymphocyte subset composition in rat inguinal lymph nodes, determined by double or triple staining, using fluorochrome-conjugated monoclonal antibodies followed by flow cytometry analysis. (a) Tαβ, Tγδ, B, natural killer (NK) and natural killer T (NKT) lymphocyte percentages. (b) T-helper (Th) and T-cytotoxic (Tc) cell percentages in the T-cell population. (c) Th2, activated Th (Tact) and regulatory Th (Treg) percentages in the Th subset. (d) CD8+ and CD8− cell percentages in NK lymphocytes. Values are means, with their standard errors represented by vertical bars (n 9–12). * Mean values were significantly different from those of healthy animals fed a standard diet (REF, ![]() ) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (
) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (![]() ); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
Although the arthritic process did not modify the proportion of Th and Tc cells, both cocoa diets produced a decrease in the Th-cell proportion in ILN (P < 0·05; Fig. 4(b)).
On the other hand, neither arthritis nor the cocoa diets changed the Th2 cell profile in this tissue (Fig. 4(c)). Proportion of regional Treg cell was not affected by the arthritic process or by the cocoa diets (Fig. 4(c)). In contrast, the proportion of Tact lymphocytes in arthritic animals increased significantly in ILN (P < 0·05).
With respect to the NK cell population (Fig. 4(d)), the arthritic process increased more than twofold the CD8+:CD8− ratio in NK cells (P < 0·05). The cocoa diets avoided this marked disequilibrium in ILN (P < 0·05, C10-AA v. REF-AA).
Effect of the cocoa diet on anti-Mycobacterium butyricum antibodies in adjuvant arthritis rats
Arthritis induction, by means of heat-killed Mb, involved the synthesis of antibodies directed against mycobacteria (Fig. 5), which are absent in non-induced animals (data not shown). The serum concentration of these antibodies increased during the arthritis time course. The intake of both cocoa diets (5 and 10 %) reduced serum anti-Mb antibody synthesis, an effect that was already detected at 2 weeks after induction (P < 0·05). This inhibition was dose-dependent and continued until the end of the study.

Fig. 5 Anti-Mycobacterium butyricum (Mb) antibody concentration in serum during arthritis time course. Values are means, with their standard errors represented by vertical bars (n 11–12). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (![]() ); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
Effect of the cocoa diet on ex vivo cytokine and PGE2 secretion by splenocytes
Splenocytes obtained on day 28 post-induction from animals of all the induced groups produced IL-2 and IFN-γ after the Mb challenge (Fig. 6(a) and (b)). IL-2 secretion was higher in splenocytes from the C5-AA and C10-AA groups than those from the REF-AA group (P < 0·05; Fig. 6(a)). However, cocoa did not affect IFN-γ secretion, which was similar in all three AA groups (Fig. 6(b)).

Fig. 6 Inflammatory mediator concentrations in spleen cell supernatants. (a) IL-2 concentration in the 24 h spleen supernatant. (b) Interferon γ (IFN-γ) concentration in the 72 h spleen supernatant. (c) PGE2 concentration in the 48 h spleen supernatant. Values are means, with their standard errors represented by vertical bars (n 10–12). * Mean values were significantly different from those of healthy animals fed a standard diet (REF, ![]() ) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). NS, non-stimulated; Mb, Mycobacterium butyricum stimulated; C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (
) (P < 0·05). † Mean values were significantly different from those of arthritic animals fed a standard diet (REF-AA, □) (P < 0·05; Kruskal–Wallis and Mann–Whitney U tests). NS, non-stimulated; Mb, Mycobacterium butyricum stimulated; C5-AA, arthritic animals fed a 5 % cocoa-enriched diet (![]() ); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
); C10-AA, arthritic animals fed a 10 % cocoa-enriched diet (■).
PGE2 was secreted by splenocytes (Fig. 6(c)) from healthy and arthritic animals either without stimulus or after Mb addition. In non-stimulated conditions, PGE2 released by cells obtained from the C10-AA group was significantly lower than that from the REF and REF-AA groups (P < 0·05). However, no significant changes were observed in Mb-stimulated cells.
Discussion
In previous studies, cocoa has shown anti-inflammatory properties both in vitro and in vivo (Reference Ramiro, Franch and Castellote22, Reference Castell, Franch, Ramos-Romero and Keller31). We have previously demonstrated that cocoa-enriched diets in young and adult animals modulate the synthesis of total and specific antibodies(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13, Reference Pérez-Berezo, Ramiro-Puig and Pérez-Cano15) and also decrease the proportion of Th cells in several lymphoid compartments(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero14) in healthy conditions. On the other hand, some studies have reported the protective effect of flavonoids on arthritis models(Reference Li, Cai and Xie37–Reference Haqqi, Anthony and Gupta39), and specifically on adjuvant-carrageenin-induced arthritis in rats(Reference Guardia, Rotelli and Juarez40, Reference Rotelli, Guardia and Juárez41). All these results prompted us to study the influence of long-term cocoa diets with two different dosages in a T-cell-mediated systemic inflammation model, as in AA(Reference Pelegrí, Morante and Castellote19), a well-established severe polyarthritis chronic model that lasts at least 3 months(Reference Franch, Castellote and Castell42).
The present study shows that a cocoa-enriched diet was able to decrease the synthesis of antibodies against the pathology inducer during the progression of AA. The cocoa diet was also able to decrease the proportion of Th lymphocytes in blood and regional lymphoid tissues, which probably include cells responsible for the arthritic process. Moreover, the cocoa diet showed a tendency to modulate hind-paw swelling. The clinical evolution of AA in animals fed a 5 % cocoa-enriched diet had a tendency to reduce the severity of the process, but unfortunately, a significant diminution of the arthritic process was not achieved through the cocoa diets. These results are in contrast with those of Pelzer and co-workers(Reference Guardia, Rotelli and Juarez40, Reference Rotelli, Guardia and Juárez41), which showed flavonoid anti-inflammatory actions in a similar model. However, these studies were performed by using single flavonoids (quercetin, rutin, hesperidin and morin) administered intraperitoneally, and the AA model was induced through a rather different procedure. In addition, some of these flavonoids, such as hesperidin, morin and rutin, are not even present in cocoa, and there are controversial results about the anti-inflammatory capacity of hesperidin(Reference Rovenský, Stančíková and Rovenská43). Our results do not agree with another study that used quercetin administered orally in a rat AA model(Reference Mamani-Matsuda, Kauss and Al-Kharrat44). Nevertheless, the quercetin dosage used in that study was very high (150 mg/rat), while the quercetin concentration in a cocoa extract is just over 57 μg/g(Reference Ortega, Romero and Macià45). The lack of a clear effect in the present study could then be explained by the low proportion of quercetin in cocoa flavonoids(Reference Ortega, Romero and Macià45) and the daily intake of cocoa that was about 600 mg/100 g of rat(Reference Pérez-Berezo, Ramiro-Puig and Pérez-Cano15). In fact, the main and best-absorbed flavonoid in cocoa is epicatechin(Reference Rusconi and Conti4), a flavonoid with ascribed anti-inflammatory activities in vitro (Reference Ramiro, Franch and Castellote22, Reference Mackenzie, Carrasquedo and Delfino33).
Previous studies(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana13, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero14) performed on young healthy rats showed that a 10 % cocoa-enriched diet produced a decrease in Th proportion in the spleen and lymph nodes. Here, we have also showed that a cocoa diet reduces the Th proportion in the blood and ILN in AA rats. In consequence, it could be suggested that cocoa intake could entail reduction in the number of Th cells involved in the arthritic process(Reference Pelegrí, Morante and Castellote20). However, this effect may not be enough to abolish the activity of pathogenic cells. This suggestion is in line with the concentration of IFN-γ, a pro-inflammatory cytokine, released from the splenocyte supernatants, which were similar in the three arthritic groups. IFN-γ is a cytokine mainly produced by Tact cells that enhances the inflammatory process through macrophage activation(Reference Saha, Jyothi Prasanna and Chandrasekar46).
The effect of a cocoa diet on Treg cells is also of interest because these cells regulate immune responses(Reference Belkaid and Rouse47), and there is some evidence that patients with rheumatoid arthritis have defective Treg cell function(Reference Sarkar and Fox48). It has been recently reported that the transference of activated Treg cells to mice with collagen-induced arthritis significantly prevented the disease development(Reference Kelchtermans, Geboes and Mitera49) and also slowed the arthritic progression(Reference Morgan, Flierman and van Duivenvoorde50). In the present study, AA reduced the proportion of the Treg population in blood, but not in ILN. Interestingly, preventive consumption of a 10 % cocoa-enriched diet avoided the Treg decrease, keeping it in the healthy proportion. The increase in the Treg subset proportion in the C10-AA group could be due to an increase in IL-2 synthesis, because it has been reported that IL-2 favours Treg production(Reference Malek, Yu and Zhu51). In the present study, an IL-2 increase was observed in splenocytes from cocoa-fed rats. In any case, the enhancement of Treg induced in 10 % cocoa-fed animals was not reflected by a decrease in Tact lymphocytes, and these changes were only present in blood and not in ILN.
Another result that deserves attention is the effect of the cocoa diet on NK and NKT cells. NKT cells are immunoregulatory T lymphocytes that can promote cell-mediated immunity against tumours and infectious organisms but can also suppress the cell-mediated immunity associated with allograft rejection and autoimmune disease(Reference Godfrey, Stankovic and Baxter52). In the present study, AA increased the NKT cell percentage in ILN, and decreased that in blood. With respect to NK cells, previous studies have determined that CD8+ NK cells are more cytolytic than CD8− NK cells and this molecule helps NK cells to survive after target cell lysis(Reference Addison, North and Bakhsh53). In the present study, the NK CD8+:CD8− ratio was increased in ILN from AA animals, suggesting an activation of cytolytic function in these cells by the inflammatory process. A cocoa diet prevented this disequilibrium in regional lymph nodes but this effect was not significantly reflected in articular swelling. Nevertheless, it would be interesting to understand the role of NKT and NK cells in the AA model.
Although AA is ascribed to the cellular response(Reference Van Eden and Waksman54), antibodies against the mycobacteria were developed(Reference Franch, Castellote and Castell55). Cocoa-enriched diets were able to decrease the levels of anti-Mb antibodies in a similar way as observed in another approach(Reference Pérez-Berezo, Ramiro-Puig and Pérez-Cano15). The role of anti-Mb antibodies in the development of hind-paw swelling is negligible but a role in the late phase of arthritis has been suggested(Reference Franch, Castellote and Castell55). From the present results, it is clear that the reduction of the antibody proportion through cocoa intake was not enough to regulate the arthritic process during the first weeks (when articular inflammation increased), but it could explain the modulation of arthritic swelling at the 4th week. It is possible that cocoa accelerated the recuperation of this pathology by decreasing the anti-Mb antibody levels. In any case, the down-regulatory effects of antibodies due to cocoa intake could be more significant in autoimmune arthritis, both in experimental models (collagen-induced arthritis) and in human disease.
With respect to PGE2 release, an increase in its concentration has been reported in the urine of AA rats(Reference Honda, Fukawa and Sawabe56), just as in human rheumatoid arthritis synovial(Reference Goetzl, An and Smith57). Here, we show that a cocoa diet reduced splenocyte PGE2 production in the C10-AA group. As macrophage is the main source of PGE2 during inflammation(Reference Humes, Bonney and Pelus58), the PGE2 reduction found here correlates with previous results, showing that peritoneal macrophages from animals fed cocoa produced lower amounts of other pro-inflammatory mediators such as IL-6, TNF-α and NO ex vivo (Reference Castell, Franch, Ramos-Romero and Keller31, Reference Ramos-Romero, Pérez-Cano and Pérez-Berezo32).
From the results obtained in the present study, it can be concluded that cocoa intake reduces the Th-cell proportion and modulates some alterations induced by the arthritic process, such as a decrease in the blood Treg cell percentage and a disequilibrium in inguinal NK cells. Moreover, a cocoa diet reduces the anti-Mb antibody concentration in sera and diminishes spleen PGE2 production. These changes are not enough to significantly decrease chronic articular swelling, although a tendency to its modulation is observed at the end of the study. Finally, we can conclude that a cocoa diet channels the organism to develop an ‘anti-inflammatory environment’. Other studies need to be performed in order to establish the effect of cocoa in autoimmune arthritic models and its potential as an accompaniment of anti-inflammatory drugs.
Acknowledgements
The present study was supported by the Ministerio de Educación y Ciencia, Spain (AGL2005-002823), the Ministerio de Sanidad y Consumo (CIBER 06/02/0079) and by the Generalitat de Catalunya, Spain (SGR 2005-0083). S. R.-R. is the recipient of a fellowship from the Ministerio de Educación y Ciencia, Spain (BES-2006-13640). The authors declare they have no competing interests. S. R.-R. carried out the study, performed the statistical analysis and drafted the manuscript. F. J. P.-C. and C. C. collaborated during the in vivo part and in the discussion of the results. M. C. and A. F. designed, supervised and coordinated the study. All the authors read and approved the final manuscript. The authors would like to thank the ‘Serveis Científico-Tècnics’ of the University of Barcelona, especially Dr J. Comas, for expert assistance in flow cytometry.












