Older adults are the fastest-growing age group in Canada. Their numbers are forecast to grow from 6.2 million in 2017 to 10.4 million in 2037 (Aging with Confidence: Ontario’s Action Plan for Seniors, 2017; “Infographic,” 2019). Although these older Canadians live longer and healthier than in previous generations (Bushnik, Tjepkema, & Martel, Reference Bushnik, Tjepkema and Martel2018), their proportionate demand for health care services outstrips younger cohorts (Gibbard, Reference Gibbard2018; Maddocks, Stewart, Fortin, & Glazier, Reference Maddocks, Stewart, Fortin and Glazier2020; Vegda et al., Reference Vegda, Nie, Wang, Tracy, Moineddin and Upshur2009). In response, politicians and policy makers are searching for ways to contain health care costs incurred by seniors, including expenditures related to common disorders, such as Alzheimer’s disease dementia (Public Health Agency of Canada, 2019; Smetanin et al., Reference Smetanin, Kobak, Briante, Stiff, Sherman and Ahmad2009). Overlooked are the costs incurred by healthy older adults presenting to physicians with subjective memory complaints,Footnote 1 which may simply be signs of normal or healthy aging (Burke & Barnes, Reference Burke and Barnes2006; Jessen, Wolfsgruber, et al., Reference Jessen, Wolfsgruber, Wiese, Bickel, Mösch and Kaduszkiewicz2014; Stewart, Reference Stewart2012).
Subjective memory complaints, such as forgetting names or one’s intention when walking into a room, are common in older adults (Ossher, Flegal, & Lustig, Reference Ossher, Flegal and Lustig2013), with prevalence estimates ranging from 25–50 per cent (Jonker, Geerlings, & Schmand, Reference Jonker, Geerlings and Schmand2000). Although help-seeking related to subjective memory complaints is relatively unstudied (Stewart, Reference Stewart2012), a nationwide poll by the Centers for Disease Control and Prevention in the United States found that 11 per cent of older adults ages 65 and older reported subjective cognitive decline in the preceding 12 months over the 2015–2017 period (Centers for Disease Control and Prevention [CDC], n.d.). Of these older adults, 40 per cent reported visiting a health care professional to discuss their symptoms (Centers for Disease Control and Prevention [CDC], n.d.). If this percentage of visits were applied to just the projected increase in the number of Ontario seniors in 2021 over 2020 (97,189 older adults), it would translate to approximately 4,400 physician visits and an estimated cost of $4,062,102.Footnote 2 Such money could be well spent, as some research has found that consultations for subjective memory complaints can inform objective determinations of mild cognitive impairment (MCI) and Alzheimer’s disease in prodromal stages (Crumley, Stetler, & Horhota, Reference Crumley, Stetler and Horhota2014; Jessen, Amariglio, et al., Reference Jessen, Amariglio, van Boxtel, Breteler, Ceccaldi and Chételat2014; Pakzad et al., Reference Pakzad, Bourque, Tahir, Bhalla, French and Savoie2017; Petersen et al., Reference Petersen, Doody, Kurz, Mohs, Morris and Rabins2001; Reisberg & Gauthier, Reference Reisberg and Gauthier2008). Yet, other studies find little diagnostic value of these complaints if they are not associated with objective functional decline, as far as their ability to predict cognitive impairment (Comijs, Deeg, Dik, Twisk, & Jonker, Reference Comijs, Deeg, Dik, Twisk and Jonker2002; Eichler et al., Reference Eichler, Thyrian, Hertel, Wucherer, Michalowsky and Reiner2015; Geerlings, Jonker, Bouter, Ader, & Schmand, Reference Geerlings, Jonker, Bouter, Ader and Schmand1999; Harwood, Barker, Ownby, Mullan, & Duara, Reference Harwood, Barker, Ownby, Mullan and Duara2004; Howieson et al., Reference Howieson, Mattek, Dodge, Erten-Lyons, Zitzelberger and Kaye2015; Jonker et al., Reference Jonker, Geerlings and Schmand2000; Jorm et al., Reference Jorm, Butterworth, Anstey, Christensen, Easteal and Maller2004, Reference Jorm, Christensen, Korten, Henderson, Jacomb and Mackinnon1997; Menon & Larner, Reference Menon and Larner2010; Mitchell, Reference Mitchell2008; Schofield et al., Reference Schofield, Marder, Dooneief, Jacobs, Sano and Stern1997). A critical factor in the relationship between subjective memory complaints and progressive cognitive decline may be the multimorbidity of chronic (e.g., two or more) conditions (Hill et al., Reference Hill, Bhargava, Brown, Kim, Bhang and Mullin2021). However, those most at risk of eventual neurodegenerative decline may not even report their subjective memory complaints to family members or physicians (Graham, Kunik, Doody, & Snow, Reference Graham, Kunik, Doody and Snow2005; Jonker et al., Reference Jonker, Geerlings and Schmand2000; Lavery, Lu, Chang, Saxton, & Ganguli, Reference Lavery, Lu, Chang, Saxton and Ganguli2007; Mitchell, Reference Mitchell2008; Pakzad et al., Reference Pakzad, Bourque, Tahir, Bhalla, French and Savoie2017; Waldorff, Rishøj, & Waldemar, Reference Waldorff, Rishøj and Waldemar2008).
In light of demographic trends, options for helping older adults effectively manage their subjective memory concerns in the community before they reach the clinic could significantly reduce Canadian health care costs (Verity, Kirk, O’Connell, Karunanayake, & Morgan, Reference Verity, Kirk, O’Connell, Karunanayake and Morgan2018; Wiegand, Troyer, Gojmerac, & Murphy, Reference Wiegand, Troyer, Gojmerac and Murphy2013). One proven option is memory intervention programs that provide education about typical versus atypical memory changes in aging, strategy training to maximize everyday memory, and coaching to optimize brain-healthy lifestyle behaviours (Frankenmolen et al., Reference Frankenmolen, Overdorp, Fasotti, Claassen, Kessels and Oosterman2018; Hudes, Rich, Troyer, Yusupov, & Vandermorris, Reference Hudes, Rich, Troyer, Yusupov and Vandermorris2019). An outcome of these interventions is a decreased intentionality of healthy older adults to seek medical help for subjective memory complaints (Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013), possibly because they help normalize common memory complaints, increase overall health literacy, and reduce anxiety about more serious concerns such as Alzheimer’s disease (Hudes et al., Reference Hudes, Rich, Troyer, Yusupov and Vandermorris2019; Norman et al., Reference Norman, Woodard, Calamari, Gross, Pontarelli and Socha2020). By reducing unnecessary physician visits, such programs may also deliver cost savings to Canada’s health care system and help ensure that those who see a physician for subjective memory complaints are educated about normal age-related memory loss.
From a public health perspective, this study highlights how memory interventions could be cost-effective and how innovative options help address older adults’ greater interaction with and dependence upon the health care system in Ontario (Vegda et al., Reference Vegda, Nie, Wang, Tracy, Moineddin and Upshur2009). As cognitive intervention exercises undergo greater scrutiny and study (Jaeggi, Pahor, & Seitz, Reference Jaeggi, Pahor and Seitz2020), there is little objective evidence about the costs and potential paybacks of related community-based healthy aging programs. Our goal was to report the understudied financial benefits of healthy aging programs delivered in a health care outpatient or community setting. We quantified these savings using a cost-benefit analysis (Evers, Van Wijk, & Ament, Reference Evers, Van Wijk and Ament1997) of an intervention based in Ontario (in order to maintain an alignment with one province’s physician schedule-of-benefits). Since physicians throughout Canada earn fees on a similar per patient/per fee basis, the model could be adopted throughout the country. However, specific regional/provincial factors would have to be taken into account, including the relative rates of subjective memory complaints and local variations in physician help-seeking rates for these complaints (e.g., Pakzad et al., Reference Pakzad, Bourque, Tahir, Bhalla, French and Savoie2017; Verity et al., Reference Verity, Kirk, O’Connell, Karunanayake and Morgan2018).
Methods
Cost-Benefit Analysis
We used the core steps of a cost-benefit analysis, one of the four generally accepted economic evaluations for health care interventions (Drummond, Reference Drummond2015; Snell, Reference Snell2011). These analyses estimate the costs and benefits in the same unit of money, so that decision makers can have objective evidence on which to base decisions on the efficiencies of a program (Drummond, Reference Drummond2015; Evers et al., Reference Evers, Van Wijk and Ament1997). The costs are straightforward in the present analysis: estimated administrative and program expenses related to presenting the intervention. The benefits are dollars saved (Prigatano & Pliskin, Reference Prigatano and Pliskin2003), specifically those pertaining to physician billings and related direct diagnostic fees. These benefits are predicted to be expenditures averted by older adults who do not present to physicians with normal subjective memory concerns. In terms of a cost analysis using physician billings within Canada, a precedent can be found in the examination done by Trachtenberg and Manns (Reference Trachtenberg and Manns2017). These physician-researchers tabulated physician input costs related to end-of-life care to “calculate a predicted range of savings associated with the implementation of medical assistance in dying” (Trachtenberg & Manns, Reference Trachtenberg and Manns2017, p. E101).
Costs: intervention considered
The intervention considered for this analysis is a 10-hour, five-week group initiative, the Memory and Aging Program (Troyer & Vandermorris, Reference Troyer and Vandermorris2012). It was developed and implemented at a tertiary geriatric care centre in Toronto. In recent years, it has also been offered in other health care settings by providing train-the-trainer workshops and disseminating resource materials to community-based facilitators. A hands-on, multidimensional intervention, the program targets older adults with age-normal memory changes and no functional decline (Troyer, Reference Troyer2001). Evidence has found that this intervention leads to behavioural changes in participants (Vandermorris, Au, Gardner, & Troyer, Reference Vandermorris, Au, Gardner and Troyer2020; Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013). These behaviours orient participants to “brain healthy” ways of dealing with everyday situations and environments. Throughout the intervention, an emphasis is placed on the initiation and maintenance of proven memory strategies (Vandermorris et al., Reference Vandermorris, Au, Gardner and Troyer2020). Typical program graduates – ages 60 to 79, university-educated, cognitively normal, and self- or physician-referred – report increased knowledge and confidence regarding their cognitive abilities (Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013). Perhaps due to the knowledge they gain about normal age-related memory changes, participants declare less intentionality to visit a physician to discuss subjective memory concerns; this evidence comes from a randomized controlled trial (RCT) using a waitlist control group (Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013). In light of the evidence that it takes 3.3 attendees to find a significant disinclination of one healthy older adult to seek unnecessary medical care (Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013), we used this value (rounded to three) as the number needed to treat (NNT) to realize the benefits described below.
Sensitivity analysis. In order to test the general parameter sensitivity of the memory intervention using plausible values for a critical parameter (Levin, McEwan, Belfield, Bowden, & Shand, Reference Levin, McEwan, Belfield, Bowden and Shand2018), we also ran additional testing using NNTs of 5 and 7. The higher NNT (7) was based on recent findings from an RCT conducted by Vandermorris et al. (Reference Vandermorris, Au, Gardner and Troyer2022, unpublished raw data). We also selected a midpoint number (5) between the lower NNT of 3 and the higher NNT of 7.
Benefits: Potential Savings Pathways
For this cost-benefit analysis, we considered four different screening scenarios (I, II, III, IV) for the baseline cost savings (i.e., benefits). These scenarios were developed with reference to practice guidelines and in consultation with clinician-scientists and specialists in internal medicine and geriatric medicine at Baycrest Health Sciences. The physicians featured in these scenarios are those expected to treat older adults with possible dementia and/or neurodegenerative diseases (The Diagnostic Process: Assessments and Tests/Alzheimer Society of Canada, n.d.; Feldman et al., Reference Feldman, Jacova, Robillard, Garcia, Chow and Borrie2008; Moore, Patterson, Lee, Vedel, & Bergman, Reference Moore, Patterson, Lee, Vedel and Bergman2014; Wimo et al., Reference Wimo, Religa, Spångberg, Edlund, Winblad and Eriksdotter2013). We assigned an estimated number of patients (out of 100) proceeding along the four pathways. Following recommended best practices for decision-analytic modeling, the time horizon of this cost-benefit analysis is the lifetime of the hypothetical patients (Karnon, Brennan, & Akehurst, Reference Karnon, Brennan and Akehurst2007; Weinstein et al., Reference Weinstein, O’Brien, Hornberger, Jackson, Johannesson and McCabe2003). Similar cost-effectiveness analyses, where data are unknown or are estimated, have been done to examine a range of mental health initiatives (Evers et al., Reference Evers, Van Wijk and Ament1997), including those related to dementia caregiving (Brodaty, Reference Brodaty and Peters1991). Cost-benefit analyses using simulations of different intervention scenarios have also been used within North America to estimate broad health care costs associated with dementia in older adults (Smetanin et al., Reference Smetanin, Kobak, Briante, Stiff, Sherman and Ahmad2009).
Pathway I, family physicians. All pathways start with visits to family physicians. These doctors are the first to hear from patients or their family members about memory issues (Sadowsky & Galvin, Reference Sadowsky and Galvin2012). Although family physicians may not routinely diagnose cognitive impairments (Ahmad, Orrell, Iliffe, & Gracie, Reference Ahmad, Orrell, Iliffe and Gracie2010; Boustani, Peterson, Hanson, Harris, & Lohr, Reference Boustani, Peterson, Hanson, Harris and Lohr2003; Bradford, Kunik, Schulz, Williams, & Singh, Reference Bradford, Kunik, Schulz, Williams and Singh2009; Feldman et al., Reference Feldman, Jacova, Robillard, Garcia, Chow and Borrie2008; Koch, Iliffe, & the EVIDEM-ED project, Reference Koch and Iliffe2010; Menon & Larner, Reference Menon and Larner2010; Turner et al., Reference Turner, Iliffe, Downs, Wilcock, Bryans and Levin2004), most Canadian family physicians “strongly agree” that cognitive assessment, using such tools as the Montreal Cognitive Assessment or Mini-Mental Status Exam, is within their purview (Iracleous et al., Reference Iracleous, Nie, Tracy, Moineddin, Ismail and Shulman2009). As patients with no functional impairments and normal cognitive ability can often be managed by family physicians using these tools – as well as family histories, patient blood work, and neuroimaging results (Moore, Frank, & Chambers, Reference Moore, Frank and Chambers2018; Moore et al., Reference Moore, Patterson, Lee, Vedel and Bergman2014; Rockwood & Keren, Reference Rockwood and Keren2010) – we estimated that 40 per cent of our sample would stay within this pathway. Therefore, we considered that the majority (60%) of patients with subjective memory complaints would first visit their family physicians and then receive additional consultations from one of three specialties: neurology, geriatric medicine, or geriatric psychiatry (Rockwood & Keren, Reference Rockwood and Keren2010). Such a consideration is supported by a U.S. study that found fewer than 50 per cent of family physicians make diagnoses of dementia (Boustani et al., Reference Boustani, Peterson, Hanson, Harris and Lohr2003), preferring to use their discretion to refer suspected dementia cases (including those with subjective memory complaints) to secondary care specialists (Boustani et al., Reference Boustani, Peterson, Hanson, Harris and Lohr2003).
Pathway II, neurologists. Neurologists are the most frequent recipients of referrals from Canadian family physicians for patients with subjective memory complaints or suspicion of dementia (Chow, Binder, Smyth, Cohen, & Robillard, Reference Chow, Binder, Smyth, Cohen and Robillard2009; Feldman, Reference Feldman2009). Furthermore, in a multinational survey of caregivers of patients with Alzheimer’s disease dementia in five countries (Australia, France, Italy, Spain, and the United Kingdom), neurologists were found to be the diagnosing physician 52 per cent of the time (Wilkinson, Stave, Keohane, & Vincenzino, Reference Wilkinson, Stave, Keohane and Vincenzino2004). We estimated that 50 per cent of our cases would be seen within the neurology pathway. Additional assessment tools used by neurologists and the specialists discussed below include the Toronto Cognitive Assessment (Freedman et al., Reference Freedman, Leach, Carmela Tartaglia, Stokes, Goldberg and Spring2018) and the Dementia Rating Scale (Bellak, Karasu, & Birenbaum, Reference Bellak, Karasu and Birenbaum1976).
Pathways III and IV, geriatricians and geriatric psychiatrists. We modelled the remaining 10 per cent of our cases being assessed by geriatricians or geriatric psychiatrists (Canadian Academy of Geriatric Psychiatry – Definition of Geriatric Psychiatry, n.d.).
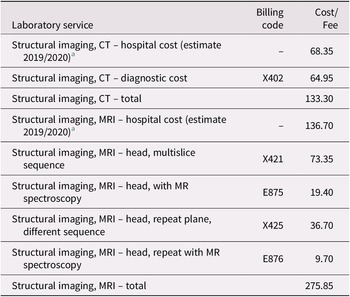
Structural neuroimaging. Costs for structural neuroimaging are challenging to estimate due to the lack of consensus within Canada on whether computed tomography (CT) or magnetic resonance imaging (MRI) neuroimaging is required to rule dementia in or out (Gauthier et al., Reference Gauthier, Patterson, Chertkow, Gordon, Herrmann and Rockwood2012). Still, it is commonly found that structural neuroimaging is indicated in most individuals with cognitive impairment in Canada (Gauthier et al., Reference Gauthier, Patterson, Chertkow, Gordon, Herrmann and Rockwood2012). A study of outpatient evaluation and treatment of dementia in an academic medical centre in Texas also found that 70 per cent of patients received MRI or CT imaging of the brain (Kalkonde et al., Reference Kalkonde, Pinto-Patarroyo, Goldman, Strutt, York and Kunik2010). We assumed that 50 per cent of patients would get a referral for CT imaging from a family physician. In contrast, almost every visit to a neurologist, geriatrician, or geriatric psychiatrist would receive a referral for either structural neuroimaging or neuropsychological testing (Gauthier et al., Reference Gauthier, Patterson, Chertkow, Gordon, Herrmann and Rockwood2012).
Results
Costs
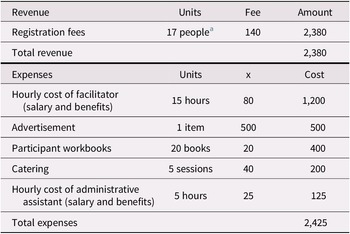
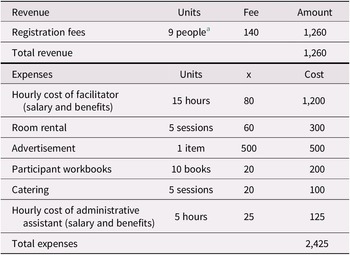
Administrative and supervisory costs for the intervention program we studied vary from site to site. In outpatient health care settings, it is estimated that approximately $2,425 is spent per 20-person session (a “full group”) in staffing, advertising, materials, and catering (Table 1a). These expenses, which are almost offset by participant program fees of $140 per attendee (as not every attendee can afford to pay the full fee, we modelled fees paid for 17 of the participants), acknowledge the direct costs related to the organization, supervision, and presentation of these programs. Because the program has been run with fewer than 20 participants and at sites where room rental fees must also be costed, we have included expenses for 10-person sessions (a “half group”; see Table 1b). For half-group sessions, we anticipated receiving registration fees for nine participants. This additional modelling, which is also applied to the sensitivity analysis, helps test parameter uncertainties associated with cost inputs.
Table 1 (a). Revenue and expenses for a group memory intervention (full group)
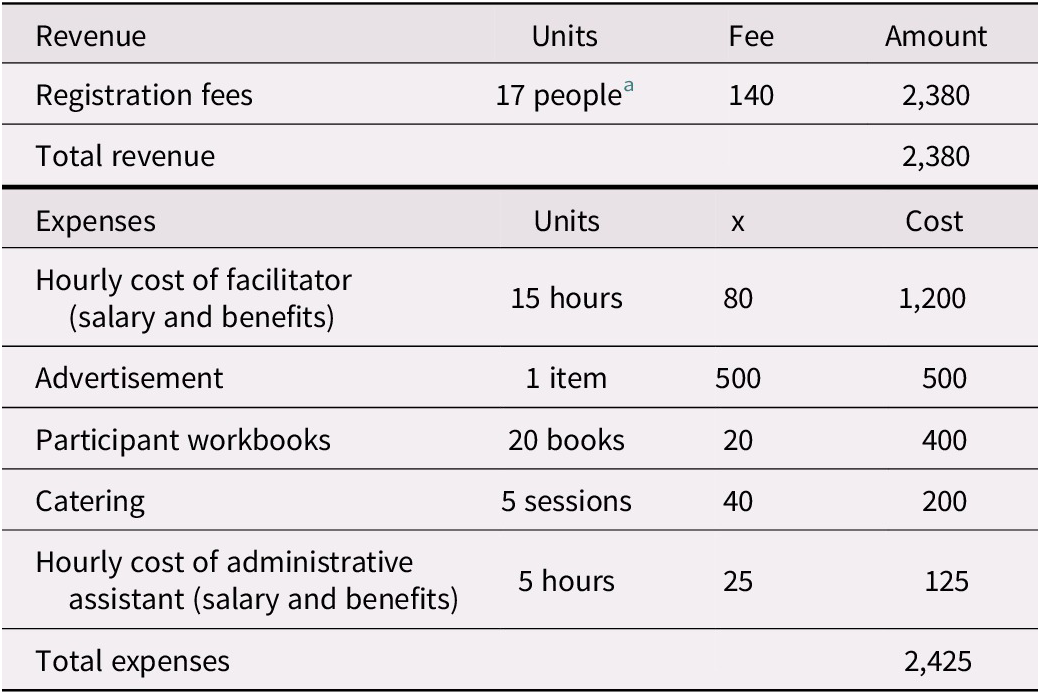
Notes. Revenue and expenses as implemented at Baycrest Health Sciences.
a Not all participants are able to pay the registration fee. We have estimate that 17 out of 20 would be able to pay the full fee, although this percentage varies across sessions.
Table 1 (b). Revenue and expenses for a group memory intervention (half group)
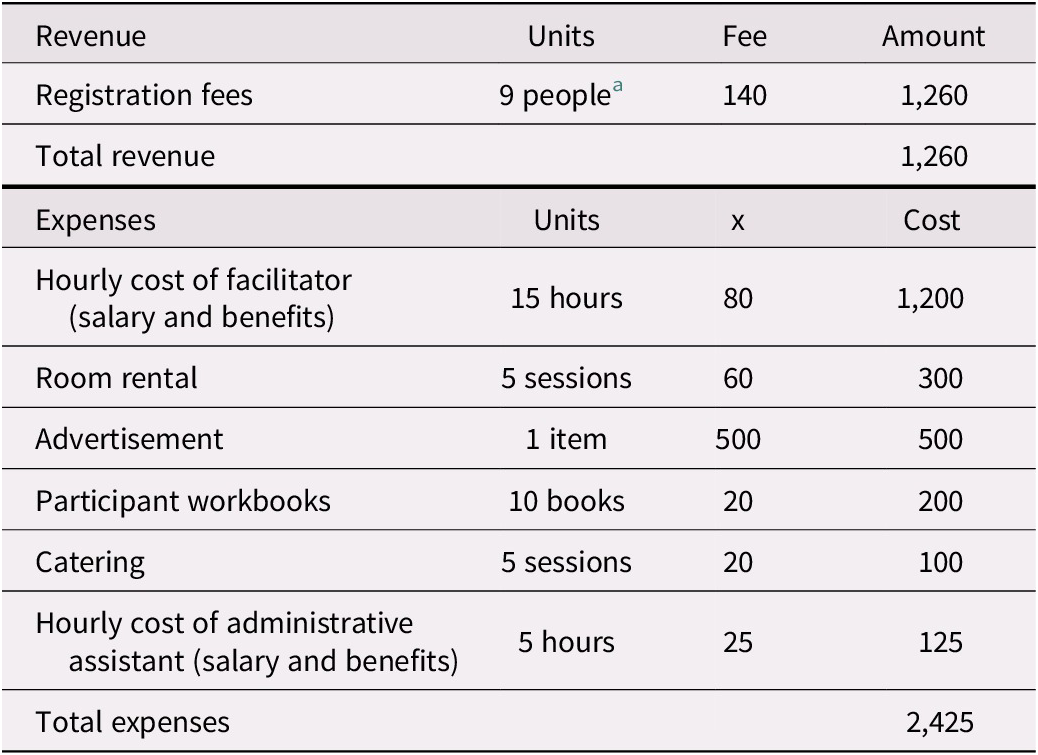
Notes. Potential revenue and expenses as implemented at non-Baycrest sites.
a Not all participants are able to pay the registration fee. We have estimate that 9 out of 10 would be able to pay the full fee, although this percentage might vary across sessions.
Benefits
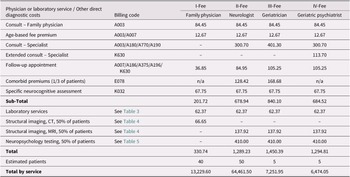
Table 2 presents the benefits (estimated costs) of a hypothetical population of 100 Ontarians presenting with subjective memory complaints to primary and secondary care physicians along four diagnostic pathways. These estimates are based on Canadian best practices for treating older adults with possible dementia or neurodegenerative diseases (Feldman et al., Reference Feldman, Jacova, Robillard, Garcia, Chow and Borrie2008; Feldman, Reference Feldman2009; Gauthier et al., Reference Gauthier, Patterson, Chertkow, Gordon, Herrmann and Rockwood2012) and align with similar costing exercises in Sweden (Jedenius, Wimo, Strömqvist, Jönsson, & Andreasen, Reference Jedenius, Wimo, Strömqvist, Jönsson and Andreasen2010; Wimo et al., Reference Wimo, Religa, Spångberg, Edlund, Winblad and Eriksdotter2013). Under the four scenarios outlined in Table 2, the estimated total cost for these 100 patients is $91,417.10 ($13,229.60 + $64,461.50 + $7,251.95 + $6,474.05). The average cost per patient being evaluated for subjective memory complaints in this simulation would be $914.17. In light of the evidence that it takes approximately three program attendees to find a significant disinclination of one healthy older adult to seek unnecessary medical care for normal age-related memory changes (Wiegand et al., Reference Wiegand, Troyer, Gojmerac and Murphy2013), we calculate that the intervention provides a benefit to the health care system of approximately $914.17 for every three individuals enrolled. Following this calculation, a typical group of 20 attendees saves approximately $6,094.47 [(20 ÷ 3) × 914.17].
Table 2. Estimated Costs for a Hypothetical Population of 100 Help-seeking Ontarians with Subjective Memory Complaints
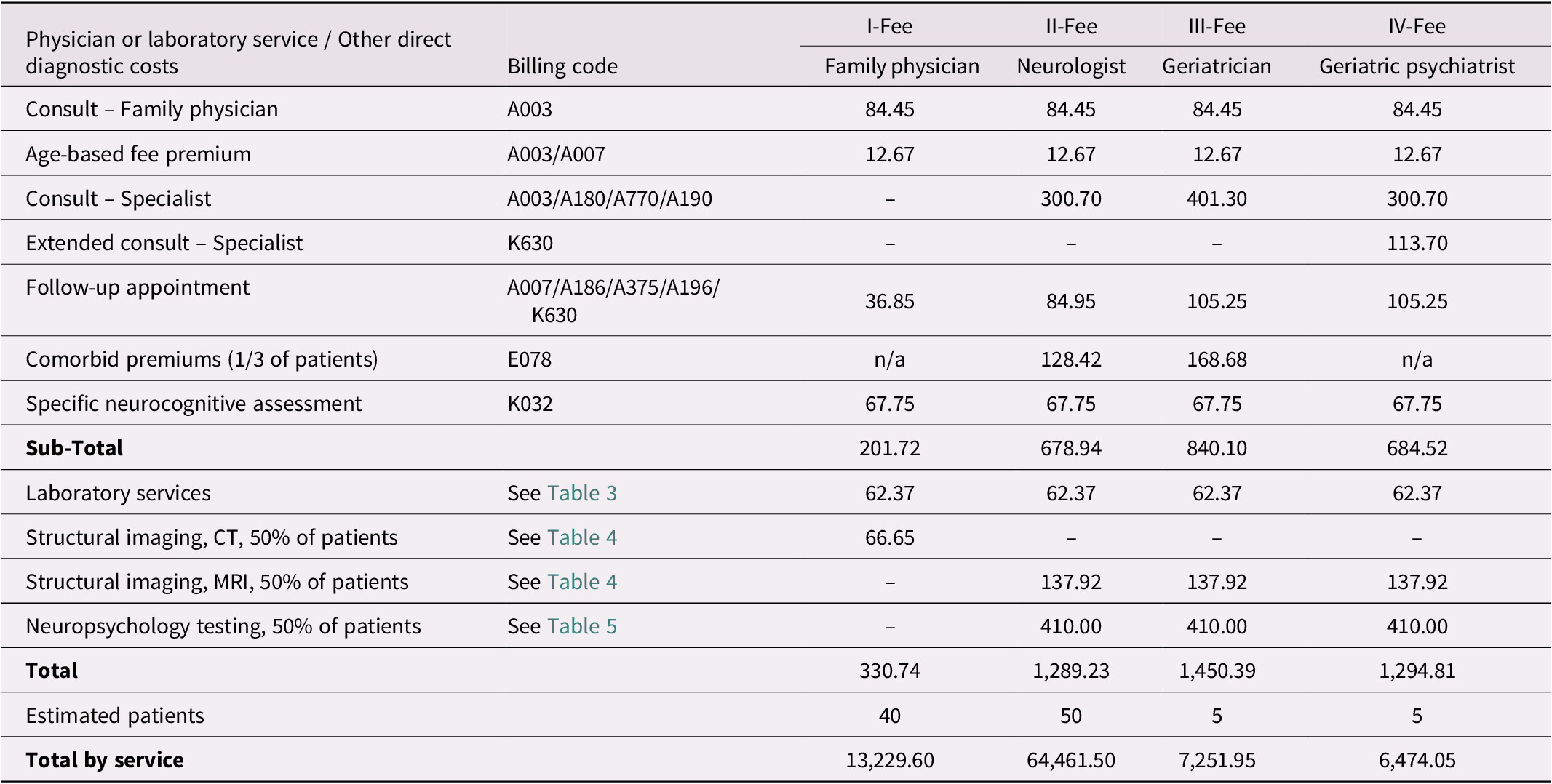
Notes. Billing codes and fees are those assigned for each physician or laboratory service in schedules under Regulation 552 of the Health Insurance Act (Ministry of Health and Long-Term Care, 2020b; Ministry of Health and Long-Term Care, 2020a). Specific neurocognitive assessments (K032) may include the short form of the Behavioral Neurology Assessment or the Dementia Rating Scale.
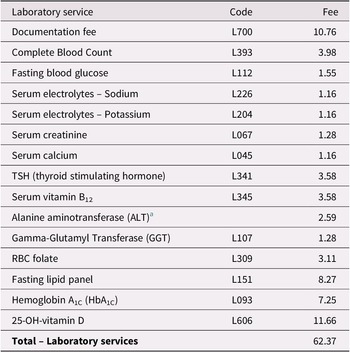
Table 3. Estimated Laboratory Services for Ontarians With Subjective Memory Complaints
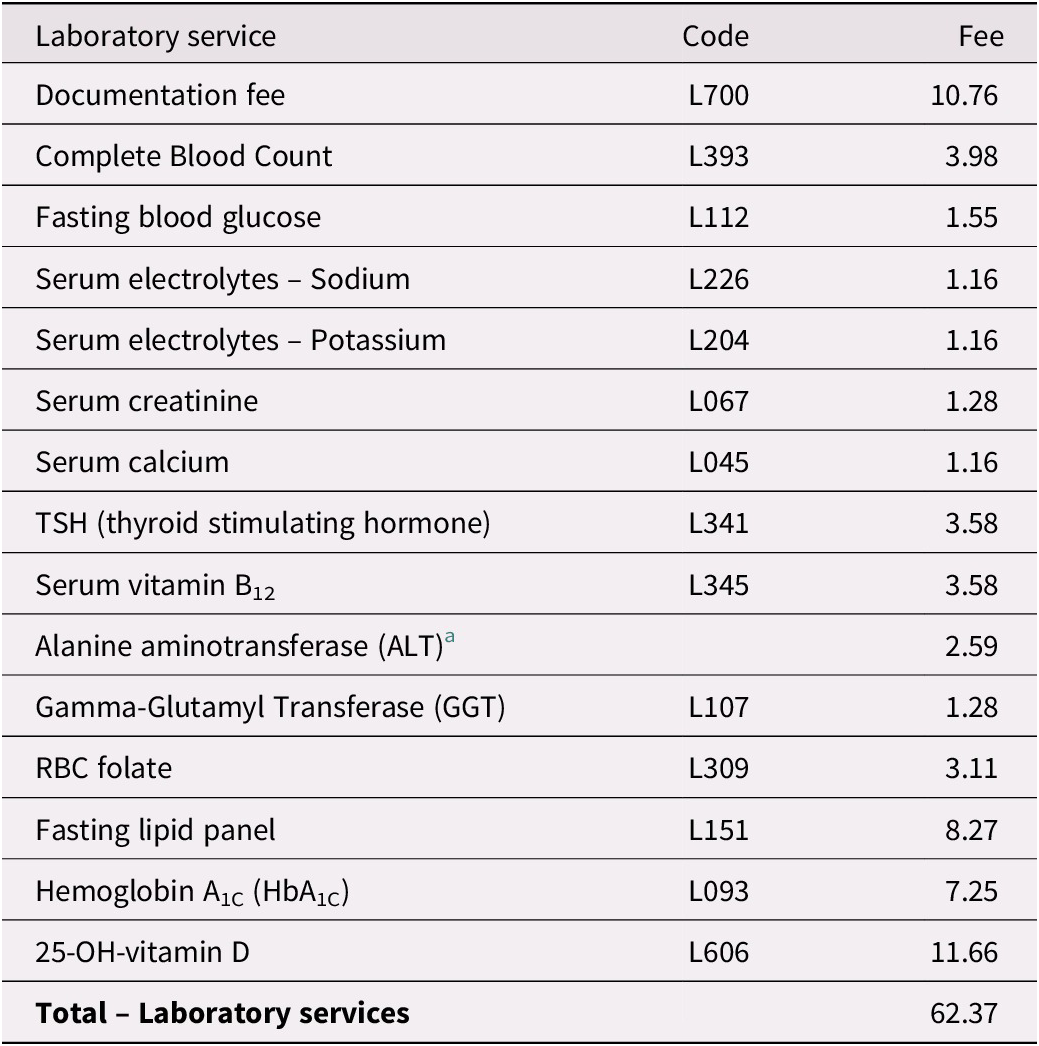
Notes. Billing codes and fees are those assigned for each laboratory service in a schedule under Regulation 552 of the Health Insurance Act (Ministry of Health and Long-Term Care, 2020a).
a ALT testing cost taken from www.lifelabs.com/Documents/files/AssessmentPanels_Summary.pdf.
Table 4. Estimated Structural Imaging Costs
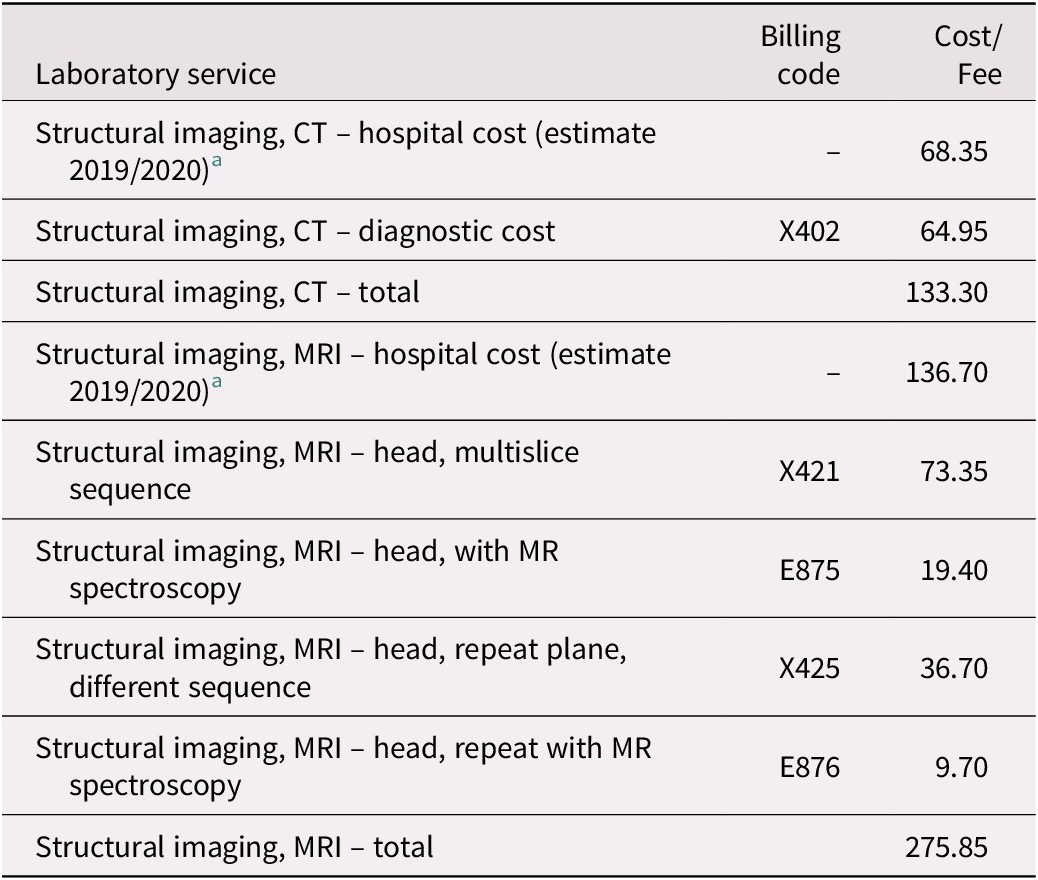
Notes. Billing codes and fees are those assigned for each service in a schedule under Regulation 552 of the Health Insurance Act (Ministry of Health and Long-Term Care, 2020b).
a Estimated hospital costs are per unit averages for MRI ($128) and CT ($64) scanning services as described in the 2017/2018 annual report of the Auditor General of Ontario (Office of the Auditor General of Ontario, 2018). These estimated hospital costs were then inflated from 2017/2018 to 2019/2020 using the annual average increase in hospital funding for the two fiscal years, or 4.7% and 2.0%, respectively (Financial Accountability Office of Ontario, 2019; “Published Plans and Annual Reports 2019-2020: Ministry of Health and Long-Term Care,” 2019).
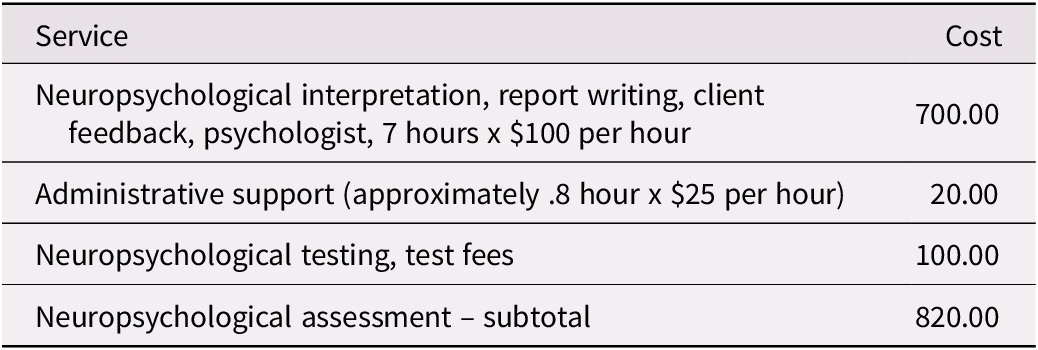
Table 5. Estimated Neuropsychological Assessment Costs
Benefit-to-Cost Ratio
A calculation of the benefit-to-cost ratio of the Memory and Aging Program is detailed in Table 6. Two ratios are presented: one for a typical, full-group session of 20 participants and another for a half-group session of 10 participants. The full-group calculation shows that the estimated benefit-to-cost ratio for a typical session is 135.43. The half-group analysis results in a benefit-to-cost ratio of 2.62. In either case, the ratios are greater than 1.0, meaning the benefits outweigh the costs, and the program is efficient (Fuguitt, Reference Fuguitt1999).
Table 6. Calculation of the Benefit-to-Cost Ratio of the Memory and Aging Program
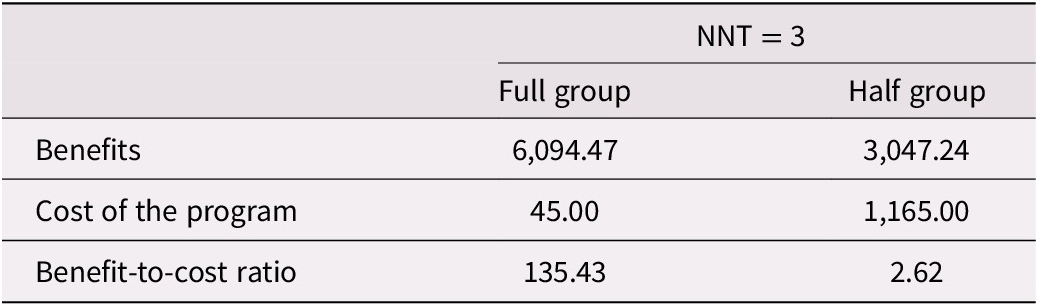
Note. Estimates for one session run at Baycrest Health Sciences with 20 participants (full group) or at an alternative site with 10 participants (half group). Half group sessions also include costs for a room rental.
Sensitivity Analysis
Table 7 provides calculations of the benefit-to-cost ratio using the higher NNT values relating to memory strategy use for program participants (Vandermorris et al., Reference Vandermorris, Au, Gardner and Troyer2020). Again, two ratios are presented: one for a full group and another for a smaller half-group. The full-group calculation shows that the estimated benefit-to-cost ratio for a typical session ranges from 81.26 (for NNT = 5) to 58.04 (for NNT = 7). The half-group analysis results in a benefit-to-cost ratio ranging from 1.57 (for NNT = 5) to 1.12 (for NNT = 7).
Table 7. Sensitivity analysis of benefits of the Memory and Aging Program based upon NNT related to memory strategy use
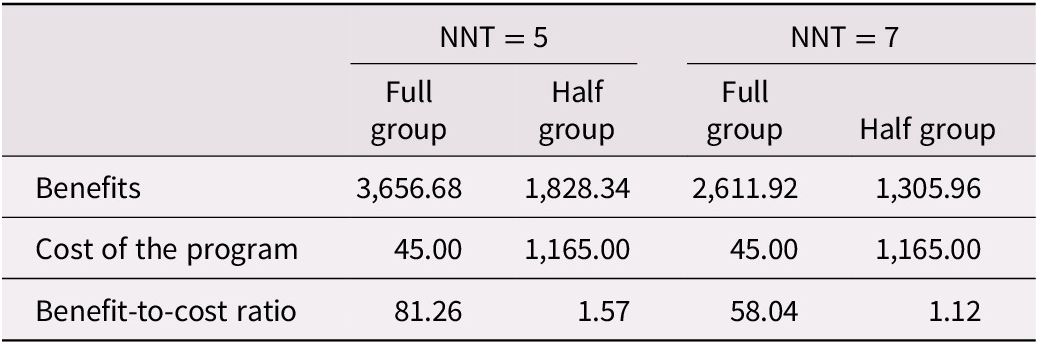
Note. Estimates for one session run at Baycrest Health Sciences with 20 participants (full group) or at an alternative site with 10 participants (half group). Half group sessions also include costs for a room rental.
Discussion
Interpretation
The 10-hour multi-component group memory intervention studied appears to be a cost-beneficial intervention. For every dollar spent on the program, a savings is accrued to one province’s health care system – in terms of health care expenditures averted – ranging from $2.62 to $135.43. This range is within the parameters of a systematic review of cost-benefit ratios of 52 public health interventions in the United Kingdom (none of them relating to memory and aging), where the ratios ran from 0.66 to 167 (Masters, Anwar, Collins, Cookson, & Capewell, Reference Masters, Anwar, Collins, Cookson and Capewell2017). The cost benefits of the program are also evident when modelling higher NNTs with smaller groups and at potential sites with additional administrative expenses.
One of our study’s underlying assumptions is that memory intervention programs raise awareness in participants about what age-related cognitive changes are normal and which are not. Therefore, these interventions contribute to attendees’ overall health literacy, or their ability to make informed, everyday decisions about health care needs (Kickbusch & Maag, Reference Kickbusch, Maag, Heggenhougen and Quah2008). Health literacy in older adults is positively correlated with cognitive health and healthier lifestyles, and a possible reduction in unnecessary physician visits and lower health care costs (Eichler, Wieser, & Bruegger, Reference Eichler, Wieser and Bruegger2009; Geboers et al., Reference Geboers, Uiters, Reijneveld, Jansen, Almansa and Nooyens2018). The educational aspect of a memory intervention program, then, can play a crucial role in directing older adults who are at the highest risk of developing MCI or dementia to seek care. In normalizing age-related memory loss, memory programs and clinics can bring attention to cognitive changes that are not part of healthy aging.
Comparison with Other Studies
There are no similar published cost-benefit analyses of memory intervention clinics or programs. The present investigation is a proof of concept as to whether such an analysis is feasible and has merit. We have proven its feasibility. We believe the research approach also has merit. It is based upon the need to estimate the potential impacts individuals with subjective memory complaints (but no diagnosable disorders) can have on health care systems. The number of worried well clients in specialist clinics throughout England has been found to range from 24–50 per cent (Stone, Pal, Blackburn, Reuber, & Thekkumpurath, Reference Stone, Pal, Blackburn, Reuber and Thekkumpurath2015). In Canada, help-seeking rates from older adults with subjective memory complaints can be inferred from studies not directly addressing this topic. For example, a survey of urban-based Canadian neurologists (n = 26) found that of 453 referrals for dementia-related symptoms (79% of which were from family practitioners), six per cent of the patients had no cognitive impairment (Chow et al., Reference Chow, Binder, Smyth, Cohen and Robillard2009). An evaluation of patients seen over three years in a memory clinic in Kitchener, Ontario, for memory complaints (e.g., word-finding problems) reported that of the 151 patients assessed by eleven family physicians and two geriatricians, about 20 per cent of the patients had normal cognitive function (Lee et al., Reference Lee, Hillier, Stolee, Heckman, Gagnon and McAiney2010). These individuals’ memory complaints were deemed attributable to factors other than cognitive impairment, such as depression or anxiety (Lee et al., Reference Lee, Hillier, Stolee, Heckman, Gagnon and McAiney2010). Indeed, a recent study in Japan of 155 older adults (mean age = 69.56) found that subjective memory complaints were associated with negative mood (e.g., depressive symptoms) rather than objective measures of cognitive decline (Kawagoe, Onoda, & Yamaguchi, Reference Kawagoe, Onoda and Yamaguchi2019). Furthermore, a 2017 study of a rural memory clinic in Saskatchewan found that 81 of 375 individuals (22%) who visited the clinic over 11 years had no identifiable neurological or neuropsychiatric disorders (Verity et al., Reference Verity, Kirk, O’Connell, Karunanayake and Morgan2018). These worried well older adults were younger, better educated, and more likely to have had previous psychiatric diagnoses or higher alcohol consumption than in a group later diagnosed with Alzheimer’s disease (Verity et al., Reference Verity, Kirk, O’Connell, Karunanayake and Morgan2018).
Extrapolating from these Canadian data, one can infer that a range of 6–20 per cent of potentially unnecessary consultations for subjective memory complaints impacts a health care system facing capacity pressures (Hallway Health Care: A System Under Strain: 1st Interim Report from the Premier’s Council on Improving Healthcare and Ending Hallway Medicine, 2019) in non-trivial ways. Although wait times for secondary care physicians specializing in diagnosing memory disorders are not tracked in Ontario, a recent report of a 4.5-year wait time to see a neurologist in Kingston (Puzic, Reference Puzic2017) may be typical of similar-sized cities and attests to the need to triage out unnecessary visits to secondary care physicians. Fortunately, memory and aging programs present family physicians with options for appropriate, low-cost interventions for older adults with subjective cognitive decline before more costly medical treatments are explored.
Future Directions
The savings accrued by this and similar initiatives are expected to grow exponentially in the 2020–2030 period, as program delivery expands into underserved areas, such as remote and rural communities and smaller cities where healthy aging programs have yet to take root. Ongoing train-the-trainer workshops and program kits for licensed health care providers will promote this growth in smaller communities. Furthermore, expansion into underserved cultural and ethnic communities will help ensure that delivery of memory and aging programs is not restricted to English-speaking individuals. Online versions of memory and aging programs will provide more options for clients, particularly during and after coronavirus disease (COVID-19) (Owens et al., Reference Owens, Ballard, Beigi, Kalafatis, Brooker and Lavelle2020), although the effectiveness of online versus in-person versions has yet to be determined. In some of the expansion areas described previously, the benefit-to-cost ratio will likely be at the lower end of the range, as host sites incur costs related to room rentals and translation of materials into different languages or formats. Yet, on economic grounds, a case will undoubtedly continue to exist for such expansion.
Limitations
Cost-benefit analyses of health care outcomes offer a narrow window to evaluate the efficacy of a health/mental health program (Prigatano & Pliskin, Reference Prigatano and Pliskin2003). They are best used in conjunction with broader outcome-based research, as cost-benefit approaches often rely on assumptions that are difficult to cost or conceptualize. Out-of-scope to the present analysis included indirect benefits, such as reduced personal and family stress, improved mental health, ability to work longer, and possible deferral of MCI/dementia to later onset, as even slowing the onset of neurodegenerative disorders can lead to substantial cost savings (Lenox-Smith, Reed, Lebrec, Belger, & Jones, Reference Lenox-Smith, Lebrec, Belger and Jones2018). Although these indirect benefits can be quantified or costed (Evers et al., Reference Evers, Van Wijk and Ament1997), such as through quality-adjusted life year analysis, such complex calculations are outside the present study’s scope.
Conclusion
In light of demographic trends, a planning concern of provincial governments is how to contain care costs related to cognitive decline in older adults. Community-based memory intervention programs that educate cognitively normal older adults about memory and aging have the potential to save health care expenditures. They do so by reducing the intentionality of older adults who are worried about their age-normal memory changes (i.e., those with subjective memory complaints) to seek traditional outlets for medical help. Using publicly available data sources, we were able to determine that these memory intervention programs are cost-effective. We did so by calculating that their benefits (in medical assessment costs avoided) outweigh typically modest program costs. Our study is a proof-of-principle that objective markers of value can be used to measure community-based memory intervention programs’ impacts. Thus, we provide a framework for future cost outcome or health literacy research, which may be informed by more robust data on the help-seeking behaviour of older adults with normal age-related memory changes.
Acknowledgements
We thank Susan Bronskill, Alita Fernandez, Dr. Jess Leah, Derrick Legere, Dr. Gary Naglie, and Dr. Gary Turner for their help and support in this project.
Conflicts of Interest
Drs. Vandermorris and Troyer have developed program materials and a train-the-trainer workshop for the Memory and Aging Program. Under Baycrest’s Intellectual Property Policy, they are eligible to receive a percentage of the royalties collected from these products.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0714980821000726.