Non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease worldwide, includes a range from steatosis to non-alcoholic steatohepatitis(Reference Rinella1,Reference Chalasani, Younossi and Lavine2) . Steatosis is a simple fat accumulation without significant inflammation or hepatocellular injury, and fat accumulation along with inflammation and evidence of hepatocellular injury refers to non-alcoholic steatohepatitis(Reference Chalasani, Younossi and Lavine2,Reference Polyzos and Mantzoros3) . The prevalence of NAFLD is estimated to be 25 % in the world(Reference Araújo, Rosso and Bedogni4), and based on a meta-analysis study, its prevalence among Iranian people is 33·95 %(Reference Moghaddasifar, Lankarani and Moosazadeh5). The global rate of the NAFLD mortality was nearly 1·27 million in 2015(Reference Estes, Razavi and Loomba6). Although the complete mechanism of NAFLD pathogenesis still remains unclear, obesity, insulin resistance and oxidative stress are the most important causes associated with pathogenesis of this disease(Reference Day and James7,Reference Polyzos, Kountouras and Mantzoros8) . Also, the association of NAFLD with elevated liver enzymes levels and dyslipidaemia has been determined(Reference Gao and Fan9). Dyslipidaemia plays an important role in the pathogenesis of NAFLD by increasing liver NEFA uptake and very-LDL (VLDL) synthesis and also by reducing NEFA oxidation and TAG export(Reference Sangouni, Ghavamzadeh and Jamalzehi10). Currently, lifestyle modifications including adherence to a healthy diet, weight loss and regular physical activity are considered as the only proven strategy for NAFLD treatment(Reference Zelber-Sagi, Godos and Salomone11,Reference Clark12) .
Garlic has been known as a therapeutic plant in various cultures, throughout history(Reference Bayan, Koulivand and Gorji13). Bioactive compounds of garlic including allicin, S-allylcysteine, ajoene, diallyl disulphide, S-allylcysteine sulphoxide and S-methylcysteine sulphoxide have caused its therapeutic properties(Reference Iciek, Kwiecień and Włodek14,Reference El-Bayoumy, Sinha and Pinto15) . According to the evidence, garlic consumption reduces the risk of CVD and cancer(Reference El-Bayoumy, Sinha and Pinto15,Reference Chan, Yuen and Chan16) . Experimental and clinical investigations have shown the beneficial effects of garlic on insulin resistance and obesity, which are linked to NAFLD pathogenesis, and as a result, garlic may help improve NAFLD(Reference Padiya, Khatua and Bagul17,Reference Soleimani, Paknahad and Askari18) . Recently, the beneficial effect of garlic on fatty liver and liver enzymes such as alanine transaminase (ALT) and γ-glutamyltransferase (GGT) has been suggested(Reference Maeda, Miki and Morihara19,Reference Kim, Kang and Roh20) . To the best of our knowledge, there is no clinical trial evaluating the effects of garlic consumption on hepatic steatosis and liver enzymes in patients with NAFLD. Also, findings related to the effects of garlic consumption on dyslipidaemia are not integrated(Reference Zeng, Zhang and Zhao21), and this issue has not been studied in patients with NAFLD. Accordingly, this clinical trial was designed to evaluate the effects of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile (as the criterion for dyslipidaemia) among patients with NAFLD.
Methods
Recruitment and eligibility screening
One hundred and ten patients with NAFLD were identified and screened consecutively at Imam Khomeini educational and medical centre in Urmia, Iran. Inclusion criteria included patients with grade 1–3 fatty liver and aged ≥18 years. Patients were excluded from the study if one of the following conditions existed: history of alcohol abuse (an average daily alcohol consumption of ≥10 g for women and ≥20 g for men), viral hepatitis, liver cancer, other liver diseases, diabetes mellitus, untreated hypothyroidism, mental diseases, kidney diseases, pregnancy, lactation, low blood pressure, taking blood pressure lowering medications, allergic to garlic and unwillingness to continue the study. Finally, ninety patients were included in the study.
Trial design
This 12-week study was double-blind, randomised controlled clinical trial. Written consent signed by participants was approved by the ethics committee of Urmia University of Medical Sciences. The participants were informed about the risks and benefits of the study and were free to drop out at any time for any reason. Patients, researcher, laboratory staff and statistician were blinded to the study groups until the end of the study. At baseline, patients were randomly divided into two groups. Randomisation lists were computer-generated by a statistician, and then participants were assigned to the study groups. A trained person randomly allocated and assigned the participants to the study groups. The treatment group received 400 mg garlic powder tablets (each coated tablet contained 1·5 mg allicin, approximately 2 g of fresh garlic) four times/d, and the control group received four placebo tablets (each 400 mg coated tablet contained starch) daily along with the usual treatment. The usual treatment for all patients was prescribing milk thistle tablets and recommending weight loss without offering any method. The study protocol was registered at the Iranian clinical trials website (IRCT20170206032417N4).
Intervention
Participants were given garlic and placebo tablets every 3 weeks. Based on the study of Lawson & Hunsaker(Reference Lawson and Hunsaker22), the effective dose of allicin for clinical trials is 3·6–7·8 mg daily. So, patients took four tablets daily, which provide 6 mg allicin. Participants took two tablets an hour before lunch and two tablets an hour before dinner, due to the reduced garlic bioavailability during protein intake(Reference Lawson and Hunsaker22). Garlic and placebo tablets were manufactured at Amin Pharmaceutical Company. Placebo and garlic powder tablets had similar appearance. The consumption of garlic powder and placebo tablets was monitored every 3 weeks. Poor compliance was defined as <80 % of expected tablets taken.
Dietary intake and physical activity assessment
In order to measure average intake of food group servings including dairy products, meats, grains, vegetables, fruits, fats and sugars, a 3-d (one weekend day and two non-consecutive weekdays) 24-h recall questionnaire was used at weeks 0, 6 and 12. In addition, the average consumption of allicin-containing plants including garlic, onion, scallion and leek at weeks 0, 6 and 12 was evaluated. Assessment of physical activity was performed at weeks 0, 6 and 12 by using the metabolic equivalent of task questionnaire.
Laboratory evaluations
Laboratory tests were performed at weeks 0, 6 and 12 to determine serum concentrations of ALT, aspartate transaminase, GGT, alkaline phosphatase (ALP) as well as total cholesterol (TC), TAG, HDL-cholesterol and LDL-cholesterol. Blood was drawn after 12 h fasting. At a speed of 3600 rpm, blood samples were centrifuged for 10 min. Serum samples were evenly poured into the microtubes, and the microtubes were immediately frozen at –80°C. ALT, aspartate transaminase, GGT, ALP as well as TC, TAG, HDL-cholesterol and LDL-cholesterol were measured by using routine enzymatic assays with commercial kits (Pars Azmoon) using an autoanalyzer (AVIDA 1800 chemistry system; Siemens). All laboratory assessments were performed in Department of Nutrition laboratory by the standard laboratory methods.
Liver ultrasonography
At the beginning and end of the study, hepatic steatosis was evaluated by abdominal ultrasound. In order to perform the assessment, participants were in fasting and supine position. The grade of steatosis was determined based on the modified criteria of Kurtz et al. (Reference Kurtz, Dubbins and Rubin23) including liver brightness, the contrast ratio of liver:kidney, blurred vessels, narrowing of the lumen of the hepatic veins and attenuation of echogenicity on ultrasonography (Philips Affiniti 50 Ultrasound). Subjects with grade 1–3 identified by ultrasound were included in the study. By using semi-quantitative criteria for steatosis, results were graded including normal: hepatic echogenicity was equal to renal cortex, grade 1: increased diffuse hepatic echogenicity along with visible periportal and diaphragmatic echogenicity, grade 2: increased diffuse hepatic echogenicity along with invisible periportal echogenicity without obscuration of the diaphragm and grade 3: increased diffuse hepatic echogenicity along with invisible periportal echogenicity and obscuration of the diaphragm. All ultrasound examinations were performed by one professional technician using the same device and criteria.
Anthropometric assessment
At the beginning of the study, the height of patients was measured by a stadiometer in standing position without shoes with an accuracy of 0·5 cm. Weight and waist circumference (WC) were measured by scale and bioelectrical impedance analyzer (In Body 770) based on standard protocols with light clothes and without shoes, respectively, at weeks 0, 6 and 12. BMI was calculated by the following formula: weight (kg)/height squared (m2).
Statistical analysis
The appropriate sample size, based on the study of Ashraf et al. (Reference Ashraf, Aamir and Shaikh24) with α = 0·05 and power = 95 %, was estimated to be thirty-seven per study group. Considering a drop-out rate of approximately 20 %, the final sample size needed was estimated to be forty-five per group. In order to finding the confounding factors, independent t test (for continuous variables) and χ 2 test (for categorical variables) were used to compare differences in general characteristics and dietary intakes between the two groups at baseline. Differences in NAFLD grade changes at the end of the study (reduction of grade or unchanged) were analysed by χ 2. To determine the effect of garlic powder and placebo on serum concentration of ALT, aspartate transaminase, GGT, ALP as well as TC, TAG, HDL-cholesterol and LDL-cholesterol, we applied a general linear model ANOVA for repeated measurements with Bonferroni post hoc analysis. In these analyses, the groups were regarded as between-subject factors and time was considered as a within-subject factor. Data were analysed with the use of SPSS version 24 (SPSS, Inc.). For all analyses, P < 0·05 was considered significant and all data are shown as mean values and standard deviations if not indicated otherwise. All of the variables had normal distribution.
Results
Characteristics of the patients
In this randomised controlled clinical trial, recruitment, screening and follow-up consecutively were conducted from August 2018 to March 2019. Ninety participants were randomly divided into two groups. During follow-up, two participants were excluded from trial due to surgery (n 1) and lost to follow-up (n 1). Eventually, eighty-eight participants completed the trial: treatment group (n 45) and control group (n 43) (Fig. 1).

Fig. 1. Flow diagram of the study participants.
Demographic and anthropometric factors
There were no significant differences between the two groups in baseline demographic characteristics (Table 1). No significant difference between the two groups was seen in physical activity (P > 0·05) during the study. Based on the 3-d 24-h food recall, no significant differences were observed in the mean intake of energy, consumed food groups and allicin-containing plants (P > 0·05) between the two groups.
Table 1. Demographic characteristics of patients with non-alcoholic fatty liver disease (NAFLD)*
(Mean values and standard deviations; numbers and percentages)

ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyltransferase; ALP, alkaline phosphatase; TC, total cholesterol; WC, waist circumference; MET-h, metabolic equivalent task hours.
* P values were computed by using independent t test for continuous variables and by χ 2 for categorical variables.
† To convert energy values from kcal to kJ multiply values by 4·184.
The anthropometric indices of patients with NAFLD are shown in Table 2. At baseline, no significant difference was observed in anthropometric measurements including weight, BMI and WC. At the end of the study, only WC in the treatment group was significantly decreased compared with the control group (P = 0·001) (Table 2). According to the significant improvement of WC at the end of the study, mean change of WC was entered as a covariate in the analysis.
Table 2. Effects of garlic powder on anthropometric indices of patients with non-alcoholic fatty liver disease*
(Mean values and standard deviations)
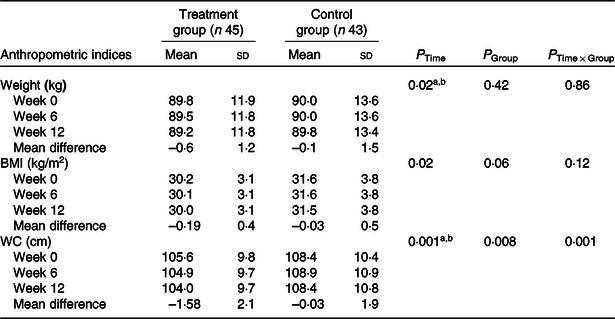
WC, waist circumference.
Post hoc test: asignificant difference between baseline and week 12; bsignificant difference between week 6 and week 12.
* P values were computed by general linear model ANOVA for repeated measurements.
Outcomes
We observed hepatic steatosis significantly reduced in the treatment group compared with the control group (P = 0·001) (Table 3). Also, ALT (P < 0·001), aspartate transaminase (P = 0·002) and GGT (P = 0·003) in the treatment group were significantly decreased compared with the control group (Table 4).
Table 3. Comparison of pre- and post-intervention grades of liver steatosis assessed by ultrasonography in patients with non-alcoholic fatty liver disease (NAFLD)*
(Numbers and percentages)
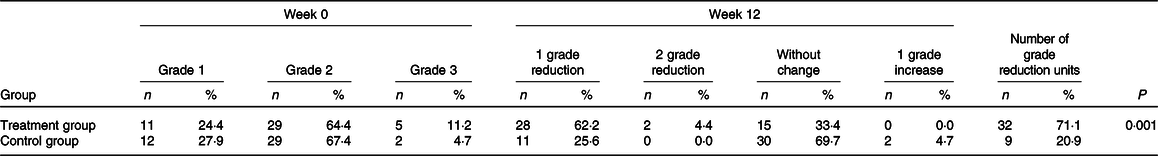
* On the basis of χ 2 test, there were no significant differences between groups with regard to baseline grades of liver steatosis (P = 0·52). P value was computed by χ 2 test to compare the difference in number of NAFLD grade reduction units between two groups at week 12.
Table 4. Effects of garlic powder on serum levels of liver enzymes and lipid profile*
(Mean values and standard deviations)
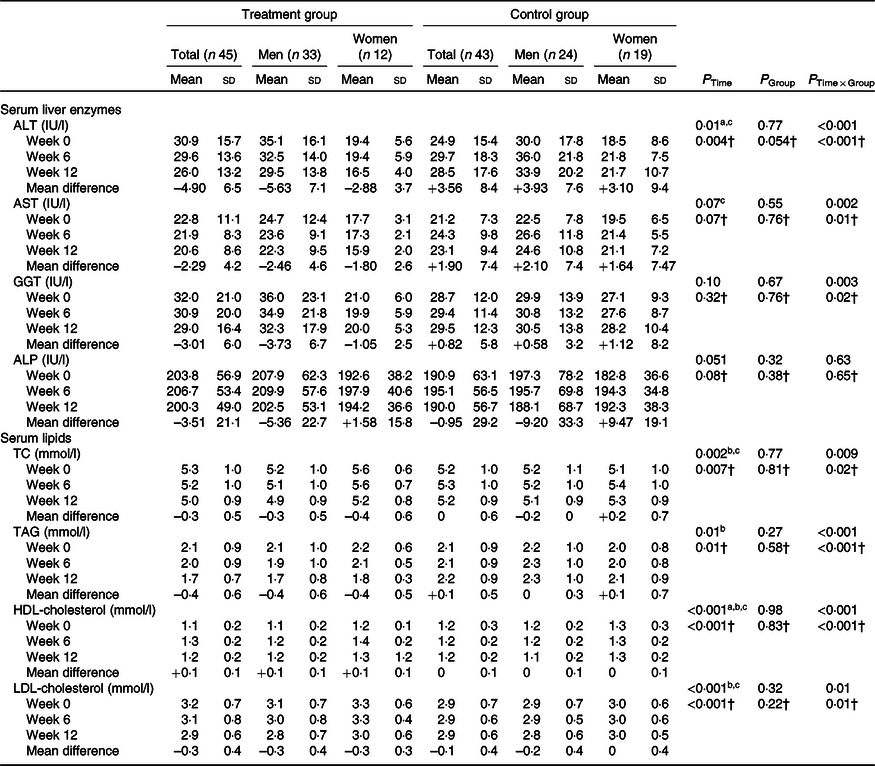
ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyltransferase; ALP, alkaline phosphatase; TC, total cholesterol; WC, waist circumference.
Post hoc test: asignificant difference between baseline and week 6; bsignificant difference between baseline and week 12; csignificant difference between week 6 and week 12.
* P values were computed by general linear model ANOVA for repeated measurements.
† Adjusted based on mean changes of WC and baseline values of parameters.
In addition, a significant decrease was observed in TC (P = 0·009), TAG (P < 0·001), HDL-cholesterol (P < 0·001) and LDL-cholesterol (P = 0·01) in the treatment group compared with the control group (Table 4).
At the end of the study, there was no significant difference between the two groups in ALP. After controlling the effect of WC as a confounder, the results remained unchanged.
Discussion
To our knowledge, this study was the first clinical trial to evaluate the effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with NAFLD. The study found that 12-week garlic powder supplementation improved hepatic steatosis, liver enzymes (except for ALP) and lipid profile as the criterion for dyslipidaemia. Possible mechanisms of the effect of garlic on improving hepatic steatosis and consequently liver enzymes could be the regulation of lipogenesis by decreasing the activity of enzymes involved in liver fat production, insulin resistance, NF-κB pathway activity and gene expression of oxidative stress markers(Reference Maeda, Miki and Morihara19,Reference Trio, You and He25) . Consistent with our study, Maeda et al. (Reference Maeda, Miki and Morihara19) demonstrated that aged garlic extract ameliorates fatty liver in a mouse model of insulin resistance. Also, in the study of Xiao et al. (Reference Xiao, Ching and Liong26) after garlic-derived S-allylmercaptocysteine intake, liver steatosis, fibrosis and NAFLD activity score significantly improved in rats with NAFLD. Usually, elevated liver enzymes are a consequence of NAFLD, though not always. In line with our results, Kim et al. (Reference Kim, Kang and Roh20) showed a significant reduction of ALT and GGT in patients with elevated levels of serum GGT. Also, Xiao et al. (Reference Xiao, Ching and Liong26) showed garlic-derived S-allylmercaptocysteine improved ALT in NAFLD rats.
As mentioned above, dyslipidaemia is an important risk factor in NAFLD pathogenesis(Reference Sangouni, Ghavamzadeh and Jamalzehi10). The beneficial effects of garlic on dyslipidaemia could be due to mechanisms such as decreasing activity of enzymes involved in liver fat production and regulation of lipogenesis, decreasing intestinal absorption of TAG and increasing adiponectin levels(Reference Ha, Ying and Kim27,Reference Lin, Wang and Lee28) . In the study of Ashraf et al. (Reference Ashraf, Aamir and Shaikh24), consumption of raw garlic resulted in a significant decrease in LDL-cholesterol and TC levels as well as a significant increase in HDL-cholesterol among type 2 diabetes patients with dyslipidaemia; however, there was no significant decrease in serum TAG levels. Also, Heidarian et al. (Reference Heidarian, Jafari-Dehkordi and Seidkhani-Nahal29) showed improvement in LDL-cholesterol, VLDL and TAG after garlic intake in hyperlipidaemic rats. Contrary to the results of our study, in the randomised controlled clinical trial of Jung et al. (Reference Jung, Park and Choi30), no significant improvement was observed in TAG, TC and LDL-cholesterol levels after black garlic extract supplementation and there was only a significant increase in HDL-cholesterol levels. Also, Turner et al. (Reference Turner, Mølgaard and Marckmann31) showed no significant improvement in TAG, TC, LDL-cholesterol and HDL-cholesterol levels in healthy subjects after garlic powder supplementation. The contradictory findings regarding the effect of garlic consumption on lipid profile can be attributed to the use of different types of garlic as well as different design of studies. In this regard, it seems that consumption of raw garlic (but not black garlic) by patients with dyslipidaemia has the best results in normalising lipid profile and no significant change was observed in lipid profile among healthy subjects. During the study, no serious side effects were reported by participants.
The most important strengths of the present study include high rate of compliance (97·7 %) and evaluation of the consumption of allicin-containing plants.
One of the limitations of the study is the use of liver ultrasonography due to its low cost, non-invasiveness and availability, despite biopsy is a ‘gold standard’ of liver steatosis assessment(Reference Saleh and Abu-Rashed32).
This clinical trial demonstrated that garlic powder supplementation for 12 weeks improves hepatic steatosis, liver enzymes and lipid profile as an indicator for dyslipidaemia. As a result, it seems that using garlic is logical in modified diet of patients with NAFLD, which is a main part of NAFLD treatment strategy. Further clinical studies with longer duration are needed to determine whether these effects of garlic will be sustained.
Acknowledgements
We would like to express our gratitude towards the Urmia University of Medical Sciences, for the facilities and financial support. We would also like to thank the patients who participated in this study.
This trial supported by the Urmia University of Medical Sciences.
The authors’ responsibilities were as follows – A. A. S. and M. A. conceived and designed the study and analysed the data; M. R. M. H. A. provided material and technical support; A. A. S. wrote the manuscript; M. A. critically revised the manuscript for important intellectual content; M. A. had primary responsibility and all authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










