The morbidity of diabetes has reached an epidemic level. In 2019, about 463 million adults between 20 and 79 years old had diabetes worldwide(1). By 2014, the estimated annual cost of diabetes-related health care has reached 825 billion international dollars(2). Patients with diabetes have a higher risk of developing CVD, which is ranked as the number one cause of death globally(1). Diabetes is also associated with a higher risk of developing other complications including kidney complications, nerve damage, eye complications, foot complications, etc.(Reference Avogaro and Fadini3). Diabetics are often treated with hypoglycaemic drugs such as sulphonylureas and metformin. However, these hypoglycaemic drugs have various side effects(Reference Lorenzati, Zucco and Miglietta4,Reference Farah, Leme and Eliaschewitz5) . Adjunctive therapy which can improve glycaemic control may be beneficial. The intake of functional foods can improve the glycaemic control for patients with type 2 diabetes mellitus (T2DM)(Reference Silva, Kramer and de Almeida6,Reference Xiong, Zhou and Wang7) . Certain functional foods may also mitigate the risk of developing T2DM(Reference Guo, Ruan and Li8).
Resistant starch, defined as the starch portion which is not digestible by human enzymes, has shown potential for improving glycaemic control(Reference Shi and Maningat9). Resistant starch is classified into five types: I, II, III, IV and V according to the different mechanisms of being resistant to human enzymes(Reference Sajilata, Singhal and Kulkarni10,Reference Hasjim, Ai, Jane, Shi and Maningat11) . Resistant starch will enter the colon and be fermented by the residing microbiota. The majority of fermentation products are SCFA, including acetate, propionate and butyrate(Reference Canfora, Jocken and Blaak12). These SCFA can stimulate the production of two insulin-related hormones, the glucagon-like peptide-1 and the peptide YY(Reference Tolhurst, Heffron and Lam13,Reference Psichas, Sleeth and Murphy14) , which were shown to promote insulin secretion(Reference Kim and Egan15,Reference Manning and Batterham16) . In addition, the consumption of resistant starch has been proposed to increase insulin sensitivity by reducing ectopic body fat and regulating adipogenesis(Reference Keenan, Zhou and Hegsted17), possibly through the action of SCFA(Reference Canfora, Jocken and Blaak12).
Previous randomised controlled trials reported both positive(Reference Gower, Bergman and Stefanovski18–Reference Maki, Pelkman and Finocchiaro27) and negative effects(Reference Penn-Marshall, Holtzman and Holtzman28–Reference Nichenametla, Weidauer and Wey31) of resistant starch on glycaemic control. In addition, the sample size of the reported randomised controlled trials was small, most of which ranged from nine to thirty subjects(Reference Gower, Bergman and Stefanovski18,Reference Bodinham, Smith and Wright20–Reference Robertson, Wright and Loizon24,Reference Penn-Marshall, Holtzman and Holtzman28,Reference Ble-Castillo, Aparicio-Trapala and Francisco-Luria30,Reference Bergeron, Williams and Lamendella32) . A meta-analysis would be helpful to provide convincing results by pooling the effects of the published randomised controlled trials results.
In the present study, we conduct a systematic review and meta-analysis including results of all populations from nineteen clinical trials in fifteen publications(Reference Gower, Bergman and Stefanovski18–Reference Johnston, Thomas and Bell33). The effects of resistant starch on all common glucose and insulin endpoints related to glycaemic control are analysed. We exclude the studies on type I resistant starch because it overlaps with studies on whole grains whose effects on glycaemic control were summarised previously(Reference Marventano, Vetrani and Vitale34,Reference Wang, Li and Chen35) . Subgroup meta-analyses are performed for pre-defined covariates including dosage of resistant starch, intervention duration, age, health status and study design. The included relevant endpoints for glycaemic control are: fasting plasma glucose, glycated Hb (HbA1c) percentage, fasting plasma insulin, four endpoints from the frequently sampled intravenous glucose tolerance test (insulin sensitivity index (SI), acute insulin response (AIR), disposition index (DI) and glucose effectiveness (SG)) and two endpoints from the homoeostatic model assessment (HOMA), HOMA-β and HOMA-insulin resistance (IR).
Methods
Search strategies
A systematic search of the studies published from inception to 6 April 2020 was performed on PubMed, Scopus and Cochrane electronic databases. The search process used the following terms: (‘diabetes’ OR ‘insulin resistance’ OR ‘insulin sensitivity’ OR ‘insulin’ OR ‘euglycemic clamp’ OR ‘glucose clamp techniques’ OR ‘intravenous glucose tolerance test’ OR ‘FSIVGTT’ OR ‘homeostasis model assessment’ OR ‘HOMA’ OR ‘metabolic syndrome’ OR ‘glycated hemoglobin A’ OR ‘HbA1c’ OR ‘glycosylated hemoglobin A’ OR ‘blood glucose’ OR ‘blood sugar’ OR ‘glucose tolerance test’ OR ‘OGTT’ OR ‘glucose intolerance’ OR ‘impaired glucose tolerance’) AND (‘resistant starch’ OR ‘amylose’). The search was restricted to studies published in English.
Inclusion and exclusion criteria
Inclusion criteria include: (1) randomised controlled trials; (2) resistant starch supplementation by itself or incorporated into food and beverage; (3) reporting blood glucose or insulin endpoints. Exclusion criteria include: (1) supplementing type I resistant starch or whole grains; (2) supplementing a mixture of resistant starch and other functional foods (e.g. fibre and polyphenol etc.) and (3) using substances other than digestible starch as controls. Two investigators (K. X. and J. W.) independently evaluated the studies based on inclusion and exclusion criteria. Any disagreement was resolved by discussion.
Quality assessment
The Jadad scale was used to assess the quality of the included human trials for the meta-analysis. The range of possible scores is 0 (bad) to 5 (good)(Reference Jadad, Moore and Carroll36).
Data extraction
The extracted basic information includes: the first author’s name, the publication year, the location of the study, the study design, the number of participants, the mean age, the health status of the participants, the duration of the interventions and the dosage of resistant starch. The extracted results include both the baseline and post-treatment values of the fasting plasma glucose concentration, HbA1c, the fasting plasma insulin concentration, SI, AIR, DI, SG, HOMA-β and HOMA-IR.
Statistical analysis
Weighted mean difference between change-from-baseline values (test v. control) was used as the effect size (ES). If baseline values were not reported, weighted mean difference between post-treatment measurements were used as the ES instead. When insufficient data are available, the effect variance was calculated using methods described by Follmann et al.(Reference Follmann, Elliott and Suh37). For a crossover design, within-study comparisons were based on paired t tests. Correlations between two measures on the same subject were estimated from P values when possible. If a correlation coefficient needs to be used, it was assumed to be 0·5 between the two measures on the same subject(Reference Elbourne, Altman and Higgins38). For a parallel design, correlations between change-from-baseline measures were assumed to be zero.
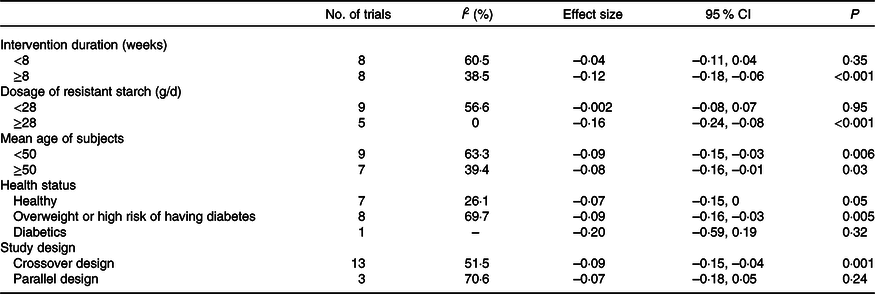
An I 2 test was used to evaluate the heterogeneity. A random effects model was used to pool the effects considering the different characteristics of the included trials (e.g. different dosages, different sexes, different health status etc.) Meta-regression and subgroup analyses were used to explore the potential source of heterogeneity including the intervention duration, the dosage of resistant starch, the mean age of subjects, the health status and the study design. In the subgroup analyses, for intervention duration, 8 weeks was used as the division point according to a methodology proposed by Chinese Nutrition Society for evidence rating(39). Dietary Guidelines for Americans 2015–2020 (8th edition)(40) recommended a dietary fibre intake of 14 g/4184 kJ (1000 kcal). Assuming a daily energy intake of 8369 kJ (2000 kcal), we used 28 g/d as the division point for the subgroup analysis by dosage. For the subgroup analysis by age, 50 years old was used as the division point because 50 years old is generally considered middle-aged.
Publication bias was evaluated using the Begg’s rank correlation test and the Egger’s regression test. Sensitivity analysis was performed by removing one trial at a time and recalculating. All statistical analyses were performed using STATA 15 software (StataCorp). The results were considered statistically significant when the P value was <0·05 unless it was noted otherwise.
Results
A total of 2441 papers were found through PubMed, Scopus and Cochrane library databases (Fig. 1). After title and abstract screening, 2407 papers were excluded and the rest thirty-four papers were subjected to full-text screening. After full-text examination, nineteen papers were excluded due to missing relevant outcome (n 7), inappropriate control (n 8) or missing detailed results (n 4). Fifteen papers were included in this study. The study by Gower et al.(Reference Gower, Bergman and Stefanovski18) consisted of four subgroups, in which two dosages (2·5 and 7·6 g/d) of resistant starch were used for each of the two groups of subjects (insulin-resistant and insulin-sensitive groups). Since the control group (0 g/d resistant starch) was shared, the two intervention subgroups for the same type of subjects were combined. The study by Bergeron et al.(Reference Bergeron, Williams and Lamendella32) was composed of two subgroups. The subjects consumed either a high-carbohydrate (51–53 % of the energy intake) or a low-carbohydrate (39–40 % of the energy intake) diet, in which a 66 and a 48 g/d dosage of resistant starch were included, respectively. The results of the low-carbohydrate group were not included because of possible confounding effects between low-carbohydrate consumption and resistant starch intake. The study by Alfa et al.(Reference Alfa, Strang and Tappia26) consisted of two subgroups, in which the effects of resistant starch on subjects with a middle age (an age of 37–47 years) and an old age (an age of 73–82 years) were evaluated separately. The results of these two subgroups were treated as two trials in this work. The study by Maki et al. was composed of four subgroups. Two different dosages (9 and 18 g/d) of resistant starch were consumed by each of the two groups of subjects (male and female groups)(Reference Maki, Pelkman and Finocchiaro27). Since the control group (0 g/d resistant starch) was shared, the two intervention subgroups for the same type of subjects were combined before the meta-analysis. The study by Nichenametla et al.(Reference Nichenametla, Weidauer and Wey31) included two subgroups (with or without metabolic syndrome), which were treated as two trials. Therefore, nineteen trials from fifteen papers were included in this study.

Fig. 1. Flow diagram of the study selection process.
Characterisations of the studies
The characterisations of the included studies are summarised in Table 1. The nineteen trials consisted of 503 subjects. Five trials used a parallel design and fourteen trials used a crossover design. The dosage of resistant starch ranged from 5 to 66 g/d. The intervention duration ranged from 2 to 12 weeks. Eight trials were performed on healthy subjects, ten trials on overweight subjects or subjects with a high risk of developing diabetes and one trial on individuals with diabetes. The Jadad scale was used to evaluate the quality of the included studies. The Jadad score of the included trials ranged from 2 to 5 as summarised in Table 1.
Table 1. Characteristics of the included trials
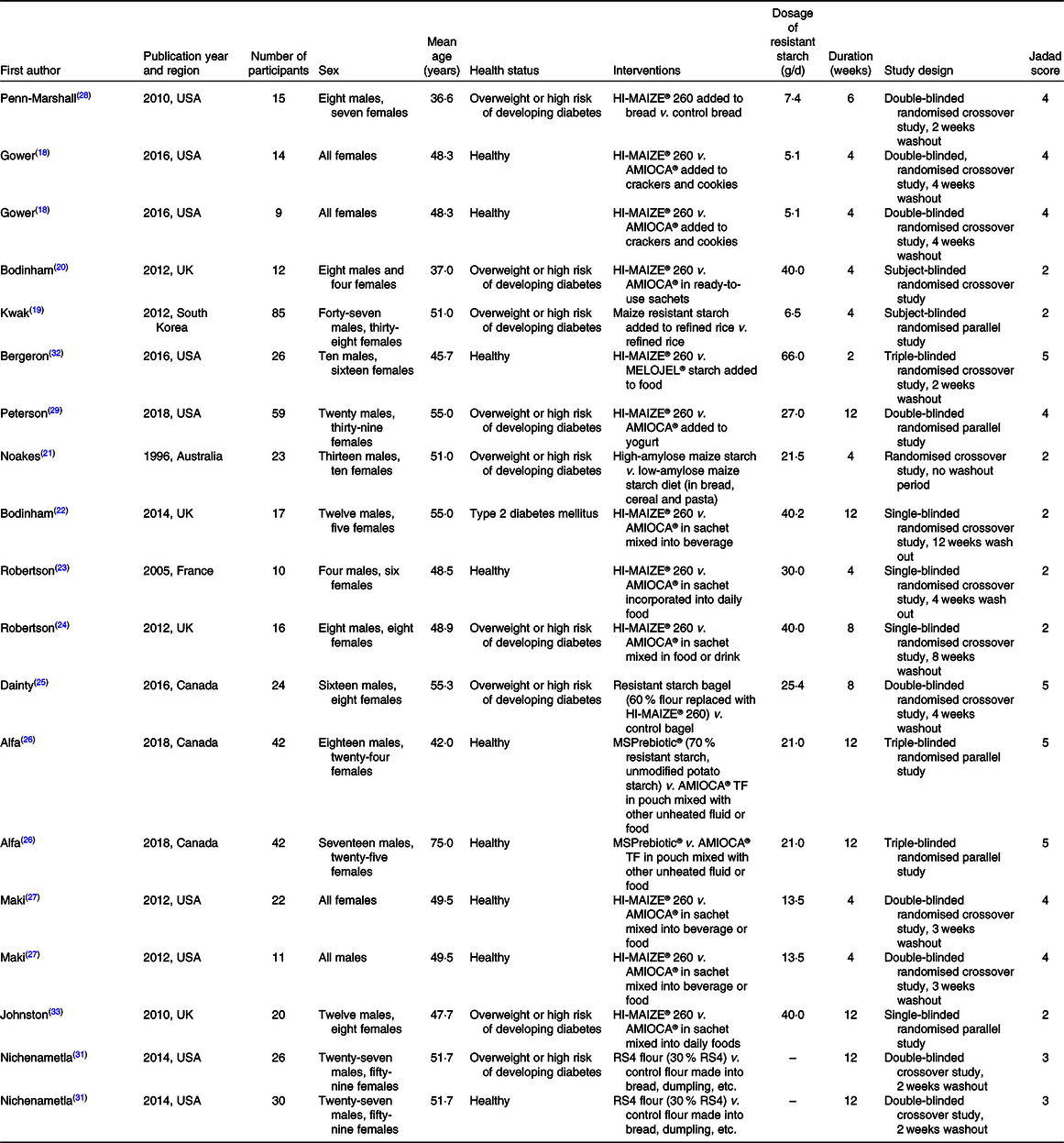
RS4, type IV resistant starch.
Effects on fasting plasma glucose
The effect of resistant starch on fasting plasma glucose was examined in sixteen trials. The results from a meta-analysis using a random effects model indicated that resistant starch had significant lowering effects on fasting plasma glucose (ES –0·09 (95 % CI –0·13, –0·04) mmol/l, P = 0·001) compared with the control (Fig. 2). There was a moderate heterogeneity among the ES of the included studies (I 2 52·7 %, P = 0·007). A subgroup meta-analysis was conducted for pre-defined covariates (intervention duration, dosage of resistant starch, mean age of subjects, health status and study design) to investigate the source of heterogeneity (shown in Table 2). Resistant starch had significant lowering effects (ES –0·12 (95 % CI –0·18, –0·06) mmol/l, P < 0·001) on fasting plasma glucose in the subgroup with more than 8 weeks of intervention, which included eight trials. The effects of resistant starch were significant when the dosage was higher than 28 g/d (ES –0·16 (95 % CI –0·24, –0·08) mmol/l, P < 0·001), which included five trials. The effects were significant for both the subgroups with a mean age of more than 50 and lower than 50. The effects were significant for the subgroup of overweight or high risk of having diabetes (ES –0·09 (95 % CI –0·16, –0·03) mmol/l, P = 0·005), which included eight trials. The effects were significant when a crossover study design was used (ES –0·09 (95 % CI –0·15, –0·04) mmol/l, P = 0·001), which included thirteen trials. No significant publication bias was detected (Begg’s test P = 1·00 and Egger’s test P = 0·30).
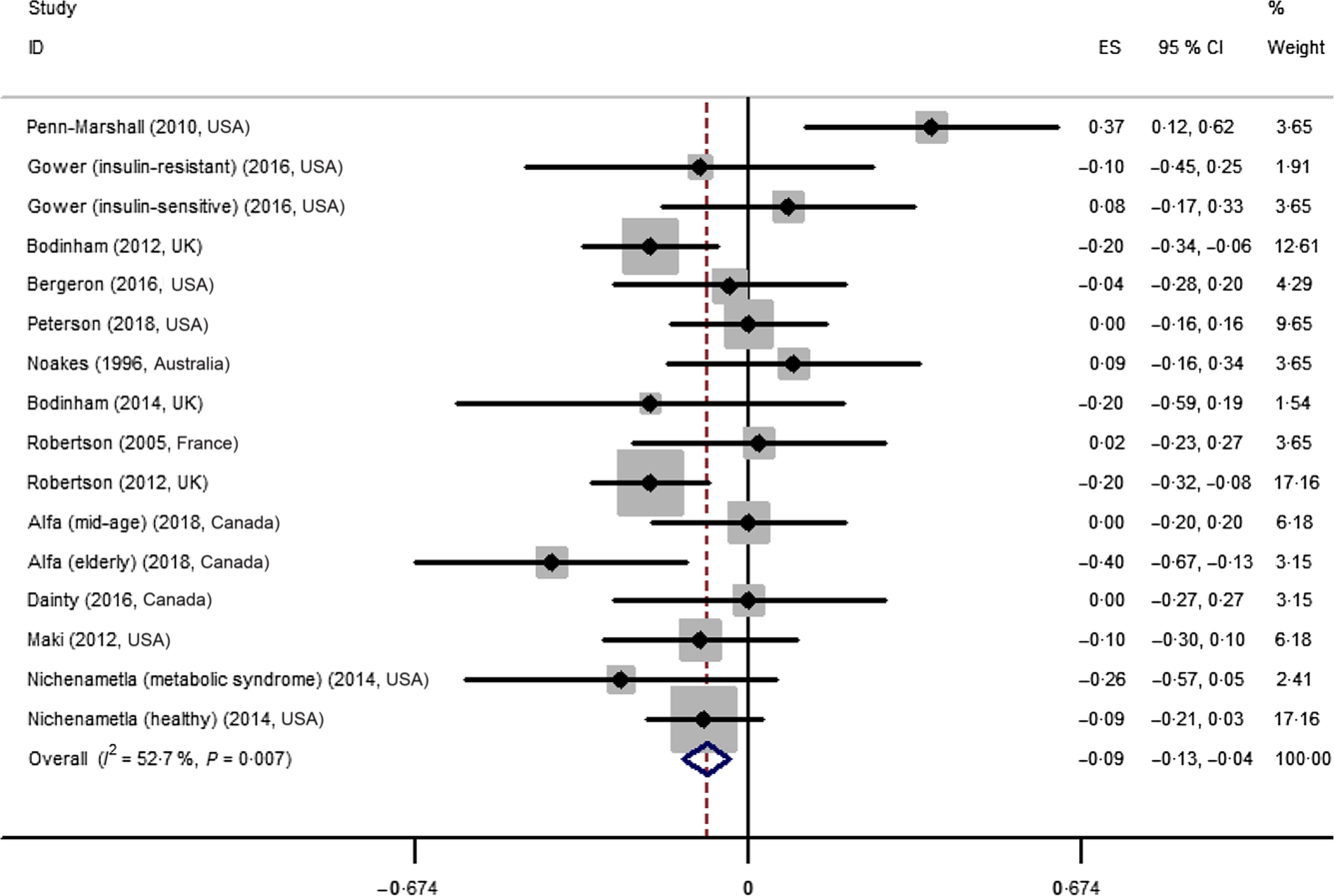
Fig. 2. Forest plot of the effect of resistant starch supplementation on fasting plasma glucose by a random effects model. The P value for the overall effect was 0·001. The diamond denotes the overall estimated effect, and the horizontal lines denote the 95 % CI. The grey bar denotes the weight percentage. ES, effect size.
Table 2. Subgroup analysis to assess the effects of resistant starch supplementation on fasting plasma glucose
Effects on HbA1c
The effect of resistant starch on HbA1c was examined in four trials. The meta-analysis using a random effects model showed no significant effect of resistant starch on HbA1c (ES –0·03 (95 % CI –0·08, 0·03) %, P = 0·39) and a high heterogeneity (I 2 77·9 %, P = 0·004) (online Supplementary Fig. A1). The limited number of trials prevented a subgroup meta-analysis. No significant publication bias was detected (Begg’s test P = 1·00 and Egger’s test P = 0·48).
Effects on fasting plasma insulin
The effect of resistant starch on fasting plasma insulin was examined in fifteen trials. The meta-analysis using a random effects model showed that the effect of resistant starch on fasting plasma insulin was not significant compared with control (ES –0·87 (95 % CI –4·03, 2·28) pmol/l, P = 0·59) (Fig. 3). A moderate heterogeneity was observed (I 2 55·0 %, P = 0·005). A subgroup meta-analysis was performed for pre-defined covariates to illustrate the potential source of the heterogeneity (shown in online Supplementary Table A1). The effect was significant when the intervention duration was more than 8 weeks (ES –8·64 (95 % CI –15·83, –1·44) pmol/l, P = 0·02), though the heterogeneity was high (I 2 70·8 %). No significant publication bias was detected (Begg’s test P = 1·00 and Egger’s test P = 0·28).
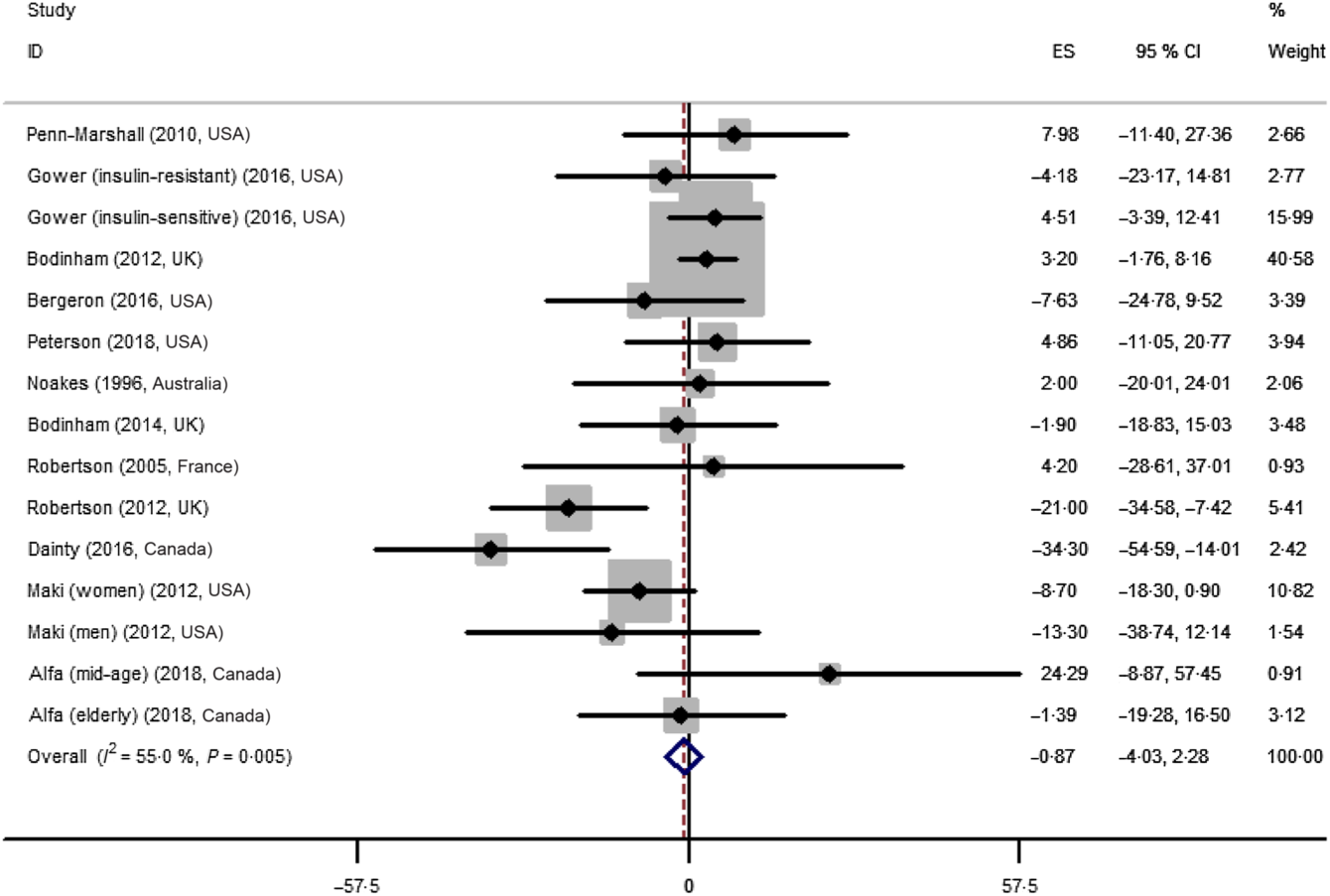
Fig. 3. Forest plot of the effect of resistant starch supplementation on fasting plasma insulin by a random effects model. The P value for the overall effect was 0·59. The diamond denotes the overall estimated effect, and the horizontal lines denote the 95 % CI. The grey bar denotes the weight percentage. ES, effect size.
Effects on insulin sensitivity index, acute insulin response and disposition index
The effect of resistant starch on SI was examined in four trials. A meta-analysis using a random effects model revealed that the effect was not significant (ES 0·30 (95 % CI –0·15, 0·75) mU/(l × min), P = 0·186) and a low heterogeneity (I 2 16·9 %, P = 0·31) (online Supplementary Fig. A2). In addition, the effect of resistant starch on AIR was investigated in five trials. A meta-analysis using a random effects model indicated that the effect was not significant (ES 91·31 (95 % CI –255·35, 437·98) pmol × min/l, P = 0·61) (online Supplementary Fig. A3). A high heterogeneity (I 2 79·4 %, P = 0·001) was observed. The publication bias was not significant (Begg’s test P = 0·22 and Egger’s test P = 0·71). The effect of resistant starch on DI was evaluated in four trials. A meta-analysis using a random effects model showed no significant effect (ES 329·51, 95 % CI –80·79, 739·82, P = 0·12) (online Supplementary Fig. A4). No heterogeneity was observed (I 2 0 %, P = 0·85).
Effects on glucose effectiveness
The effect of resistant starch on SG was examined in five trials. A meta-analysis using a random effects model showed no significant effect (ES 0·002 (95 % CI –0·003, 0·006)/min, P = 0·43) and a high heterogeneity (I 2 67·5 %, P = 0·02) (online Supplementary Fig. A5). The publication bias was not significant (Begg’s test P = 0·79 and Egger’s test P = 0·51).
Effects on homoeostatic model assessment-β and homoeostatic model assessment-insulin resistance
The effect of resistant starch on HOMA-β and HOMA-IR was evaluated in six trials and three trials, respectively. The HOMA-IR values by Alfa et al.(Reference Alfa, Strang and Tappia26) were unusually large, and these trials were excluded from the meta-analysis of HOMA-IR. A meta-analysis using a random effects model showed that the effect on HOMA-β was not significant (ES –0·06, 95 % CI –6·51, 6·40, P = 0·99) and no heterogeneity (I 2 0 %, P = 0·66) (online Supplementary Fig. A6). A meta-analysis using a random effects model revealed that the effect on HOMA-IR was significant (ES –0·33, 95 % CI –0·51, –0·14, P = 0·001) and a high heterogeneity (I 2 69·8 %, P = 0·04) (online Supplementary Fig. A7). No significant publication bias was detected for HOMA-β (Begg’s test P = 0·71 and Egger’s test P = 0·83) and HOMA-IR (Begg’s test P = 0·30 and Egger’s test P = 0·31). The limited number of trials prevented a subgroup meta-analysis to investigate the impact of covariates.
Sensitivity analysis
A sensitivity analysis was performed for the meta-analysis of the effect of resistant starch on the indices above including fasting plasma glucose, HbA1c, fasting plasma insulin, SI, AIR, DI, SG, HOMA-β and HOMA-IR (data not shown). The results indicated that all the meta-analyses were stable and omitting any one trial did not change the results, except for HOMA-IR. The removal of the trial by Robertson et al.(Reference Robertson, Wright and Loizon24) would make the effect on HOMA-IR insignificant.
Discussion
A meta-analysis including 503 subjects from nineteen human trials in the current literature was performed to resolve the controversy of the effects of resistant starch on glycaemic control. To our knowledge, the current meta-analysis investigated additional outcomes related to insulin resistance/sensitivity compared with previous meta-analyses and studied the impact of resistant starch dosage on the results. Our results indicated that the consumption of resistant starch could significantly reduce the fasting plasma glucose concentration and HOMA-IR, compared with the consumption of digestible starch. The ES was larger when the dosage of resistant starch was more than 28 g/d. In contrast, the effects of resistant starch were not significant for other insulin-related endpoints including fasting plasma insulin, SI, AIR, DI, SG and HOMA-β.
The consumption of resistant starch may reduce fasting plasma glucose by increasing insulin secretion and insulin sensitivity, possibly through the action of SCFA, as demonstrated by previous animal studies(Reference Canfora, Jocken and Blaak12,Reference Keenan, Zhou and Hegsted17,Reference Wong and Louie41) . Consistently, the current meta-analysis showed that resistant starch significantly reduced fasting plasma glucose. Our meta-analysis included results of 357 subjects from sixteen clinical trials, which should be robust. Furthermore, the subgroup meta-analyses indicated that the effect was larger when the intervention duration was more than 8 weeks or the dosage of resistant starch was more than 28 g/d. A short intervention period or small dosage may explain why some previous literature did not observe any significant effects of resistant starch on fasting plasma glucose. The consumption of resistant starch had a significant reducing effect for the fasting plasma glucose in the subgroup of overweight subjects or high risk of developing diabetes. It is possible that these subjects have higher baseline fasting plasma glucose and thus more room for improvement.
Similarly, Halajzadeh et al.(Reference Halajzadeh, Milajerdi and Reiner42) reported a significant reduction in fasting plasma glucose among patients with the metabolic syndrome by the supplementation of resistant starch (ES –0·24 (95 % CI –0·39, –0·09) mmol/l) in a meta-analysis, although their meta-analysis included two studies which used resistant dextrin as the intervention substance(Reference Aliasgharzadeh, Dehghan and Gargari43,Reference Cai, Yu and Liu44) , and two studies which used a mixture of resistant starch and other functional ingredients as the intervention substance(Reference Meng, Bai and Yu45,Reference Schioldan, Gregersen and Hald46) . Conversely, Snelson et al.(Reference Snelson, Jong and Manolas47) focused the effects of type II resistant starch (RS II) and reported a null effect of RS II on fasting plasma glucose (ES –0·03 (95 % CI 0·11, 0·05) mmol/l) in a meta-analysis. However, their meta-analysis used endpoint instead of change-from-baseline values to calculate the mean differences between the intervention and control group. After removing two type IV resistant starch studies, our meta-analysis indicated a significant reducing effect of RS II on fasting plasma glucose (ES –0·08 (95 % CI –0·14, –0·03) mmol/l) (data not shown).
The effects of resistant starch on HbA1c were not significant based on our meta-analysis. However, the results should be interpreted with caution. First, the meta-analysis only included four trials which showed a high heterogeneity (I 2 70·9 %, P = 0·008). Second, two of the four trials used an intervention period <3 months(Reference Ble-Castillo, Aparicio-Trapala and Francisco-Luria30,Reference Nichenametla, Weidauer and Wey31) . Since HbA1c represents a 3-month average blood glucose level(Reference Sharma, Panchal and Yadav48), the effects of resistant starch cannot be fully reflected with intervention duration shorter than 3 months. The meta-analysis by Halajzadeh et al.(Reference Halajzadeh, Milajerdi and Reiner42) reported a significant reduction in HbA1c in patients with the metabolic syndrome by supplementing resistant starch (ES –0·60 (95 % CI –0·95, –0·24) %). However, their meta-analysis included studies which used resistant dextrin or a mixture of resistant starch and other functional ingredients as intervention substances(Reference Aliasgharzadeh, Dehghan and Gargari43–Reference Schioldan, Gregersen and Hald46).
The meta-analysis did not show any significant effects of consuming resistant starch v. digestible starch on insulin-related indices except for HOMA-IR. However, these results should be carefully interpreted because of the limited number of trials (4–6 trials) included in the meta-analysis. In addition, the heterogeneity of the meta-analysis was high (I 2 67·5–79·4 %) for a few of these endpoints (fasting plasma insulin, AIR, SG and HOMA-IR). Future studies should continue investigating the potential effects of resistant starch on insulin secretion and sensitivity.
The present study has the following advantages. First, we strictly focused on studying the effects of resistant starch as a single intervention substance. Second, we found that the dosage of resistant starch was an important covariate in determining the effects of resistant starch. Third, in addition to HOMA endpoints, frequently sampled intravenous glucose tolerance test endpoints were also included in the present study to evaluate the effects of resistant starch on insulin sensitivity and secretion.
Limitations of the study are included below. First, many indices related to insulin secretion and insulin sensitivity were derived from models including the HOMA and the minimal model. Both models have limitations(Reference Patarrão, Wayne Lautt and Paula Macedo49–Reference Pisprasert, Ingram and Lopez-Davila51). More precise results can be obtained from the sophisticated hyperglycaemic and euglycaemic clamp method. However, the clamp method is time-consuming, requires considerable efforts from the patients and is rarely reported in human intervention trials. Second, the number of available human trials investigating the effects of resistant starch on the insulin-related endpoints was much smaller. The heterogeneity of the studies for many endpoints was high. These reduce the reliability of the meta-analysis.
The overall quality of the included trials was moderate. The median Jadad score was 4. A few trials had a low Jadad score of 2, which was mainly due to the lack of description about the randomisation method, blinding method and dropout. Future trials should include the information. The majority of the available trials included the effects of RS II on glycaemic control. With increasing research and development in resistant starch, the effects of other resistant starch types, particularly type III, IV and V resistant starch, should be more widely investigated in well-controlled human trials. In addition, the subgroup analysis revealed a greater ES by an intervention period of more than 8 weeks and a dosage of more than 28 g/d of resistant starch. Future trials should focus on investigating the effects of resistant starch under these conditions.
In summary, the current meta-analysis showed that the effects of resistant starch on fasting plasma glucose were significant with an ES of –0·09 mmol/l (P = 0·001) compared with the control. A subgroup analysis revealed that the effect was larger when the dosage of resistant starch was more than 28 g/d or the intervention period was more than 8 weeks. No significant effect was observed for insulin-related endpoints except for HOMA-IR. The current work indicates that the consumption of resistant starch is beneficial for glycaemic control. Future efforts should be put into the investigations of the effects of resistant starch types other than RS II on glycaemic control with appropriate dosage and intervention duration.
Acknowledgements
This work was funded by Qingdao University ((K. X., grant number DC1900009730) and (J. W., grant number DC1900009731)). The funder had no role in the design, analysis or writing of this article.
K. X.: conceptualisation, methodology, formal analysis, writing-original draft preparation and funding acquisition. J. W.: methodology, formal analysis, funding acquisition and writing-review and editing. T. K.: investigation. F. X.: investigation. A. M.: conceptualisation, methodology, writing-review and editing, and project administration.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003700










