The vascular endothelium of HIV-1 patients is constantly exposed to a variety of stimuli such as HIV-1 proteins and highly active antiretroviral therapy (HAART), which may cause vascular damage(Reference Kline and Sutliff1). Recent evidence has suggested an association between HIV-1 proteins, HAART and CVD, including microvascular endothelial dysfunction(Reference Currier, Lundgren and Carr2).
Vascular dysfunction observed in HIV-1 patients seems to be associated with a reduced bioavailability of NO – a decrease in NO production and/or increased NO inactivation(Reference Marincowitz, Genis and Goswami3). Since NO may influence oxygen utilisation by the cells, oxygen delivery to the skeletal muscle as well as oxygen utilisation by the muscle cells in response to exercise may be compromised in HIV-1 patients, thereby explaining the physical impairment observed in these patients(Reference Gomes-Neto, Rodriguez and Lédo4).
The consumption of beetroot juice has been proposed as a nutritional strategy due to the possible effect of the nitrate present in this food in promoting NO bioconversion(Reference Webb, Patel and Loukogeorgakis5). In brief, dietary nitrate can be reduced to nitrite in the oral cavity through bacterial nitrate reductases. The saliva-derived nitrite may be reduced to NO either in the acidic environment of the stomach or in skeletal muscles, where nitrite can also be reduced to NO under conditions of low oxygen availability and low pH – as it occurs during high-intensity exercise.
Muscle oxygen saturation may be measured non-invasively by near-IR spectroscopy (NIRS), which provides information into the ability of muscles to extract oxygen from the capillaries and the ability of microvasculature to respond to a stimulus(Reference McLay, Fontana and Nederveen6). Recently we demonstrated that a single dose of a beetroot-based gel (rich in dietary nitrate) improved muscle oxygen resaturation rate in older people(Reference Oliveira, Morgado and Conte-Junior7) and physically active individuals(Reference Alvares, Conte-Junior and Pierucci8) in response to a handgrip exercise protocol, and these improvements were accompanied by increases in urinary nitrate and nitrite(Reference Oliveira, Morgado and Conte-Junior7). However, to our knowledge, in vivo skeletal muscle oxygen saturation in HIV-infected patients after beetroot juice intake has not yet been examined.
Therefore, the main purpose of the present study was to evaluate the effect of a single dose of nitrate-rich beetroot juice (NR-BJ) on skeletal muscle oxygen saturation parameters following the completion of a handgrip exercise protocol. The analyses of salivary nitrate and nitrite and urinary nitrate were also addressed. It was hypothesised that a single dose of NR-BJ would result in increased nitrite bioconversion and would consequently improve muscle oxygen saturation parameters in response to handgrip exercise in HIV-infected patients.
Methods
Participants
Thirty-four HIV-1 patients were recruited through announcements in flyers and advertisements during community events at HIV-1 and STI assistance programmes in Macaé, Rio de Janeiro, Brazil; four patients declined to participate, and eight did not meet the inclusion criteria. Twenty-two HIV-infected patients were eligible to participate, and fifteen individuals completed all the experimental procedures (Fig. 1). Inclusion criteria were sedentary adults of both sexes, a positive diagnosis of HIV-1, undergoing regular HAART treatment for at least 1 year and aged 20–50 years; exclusion criteria were smoking, allergy to beetroot, antibiotic consumption prior to the study, osteomyoarticular diseases in the upper limbs, being physically active, known pulmonary, cardiovascular, liver and kidney diseases, diabetes, hypertension and/or acutely ill. Women were advised not to report to the laboratory during menstruation in order to eliminate potential variations in NIRS parameters(Reference Alvares, Conte-Junior and Pierucci8). All eligible participants were fully informed of the nature and purpose of the investigation and provided written consent to participate. All experimental procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and approved by the institutional ethics committee, and the trial was registered in ClinicalTrials.gov (NCT03485248).

Fig. 1. Flowchart of the total sample of individuals included in the study. NR-BJ, nitrate-rich beetroot juice; ND-BJ, nitrate-depleted beetroot juice.
Experimental procedures
The study was conducted in a randomised, double-blind, cross-over and placebo-controlled manner from October 2017 to November 2018. All participants reported to the dynamic vascular laboratory on three occasions, with at least a 1-week interval between visits. The first visit was used to explain the experimental procedures and the handgrip exercise protocol as well as collect clinical and anthropometric data (Table 1). During the second and third visits, urine samples were drawn at baseline (T0) after a 10-min period of quiet rest. After baseline sample collection, the participants received an amber bottle containing 10 ml NR-BJ or a control (nitrate-depleted beetroot juice (ND-BJ)) and were advised to hold this volume in the mouth for 5 min and then spit out all salivary volume. This procedure was performed as described previously by Kapil et al.(Reference Kapil, Haydar and Pearl9) in order to evaluate the ability of the nitrate present in the beetroot to reduce to nitrite in the oral cavity. In order to determine the load of the handgrip exercise, three maximal voluntary contraction (MVC) tests were performed using a digital handgrip dynamometer (JAMAR, Model 5030J1; Sammons Preston Roylan). Each MVC test was separated by 30 s of rest, and the highest value of MVC was recorded as the exercise workload. Afterwards, the participants consumed 140 ml of NR-BJ or ND-BJ (randomised), and muscle oxygen saturation in response to rhythmic handgrip exercise was assessed, which began approximately 150 min after the nutritional intervention (T150). The rhythmic handgrip exercise consisted of one bout of rhythmic handgrip exercise at 30 % of MVC until exhaustion, followed by 2 min of recovery. The rhythmic handgrip exercise consisted of isotonic contractions of forearm muscles at a controlled rate (sixty contractions per min, 0·5 s contraction/0·5 s relaxation, determined using a metronome) and range of motions (10-cm excursion of the pulley wire with each contraction), as previously described(Reference Oliveira, Morgado and Conte-Junior7,Reference Oliveira, Nascimento and Volino-Souza10) . A high contraction frequency cadence of exercise was chosen, since previous studies have suggested that the reduction of nitrite to NO is favoured as a high contraction frequency is performed(Reference Bailey, Varnham and DiMenna11,Reference Affourtit, Bailey and Jones12) . Exercise fatigue was determined when participants were unable to maintain the exercise rate (as contraction rhythm mismatch with metronome sound), and/or range of motions was reduced for more than five consecutive contractions. Therefore, exercise time until fatigue (ETF) was recorded from the beginning of the exercise until fatigue. Subjects were in supine position and used the dominant arm to perform the exercise. Muscle oxygen saturation was assessed 150 min after supplementation since some previous studies have shown that nitrite peak plasma occurs within 2–3 h after NR-BJ intake(Reference Wylie, Kelly and Bailey13,Reference Alvares, Conte-Junior and Silva14) . For randomisation, each participant received a numerical code (i.e. 00, 01, 02…) from laboratory staff followed by a balanced randomisation 1:1, NR-BJ:ND-BJ (ABAB). Both laboratory staff and examiners who performed all analyses, as well as the participants were blinded. Urine samples were drawn again immediately after the exercise protocol (IP-Ex) (Fig. 2). Three visits were held between 08.00 and 12.00 hours. Participants were instructed to fast for at least 8 h before each visit, restrict carbonated mineral water and caffeine consumption before the trials. They were asked to avoid high-nitrate foods and products in order not to bias nitrate and nitrite analyses, thereby receiving a list of foods as proposed by Alvares et al.(Reference Alvares, Conte-Junior and Silva14). In addition, they were advised not to brush their teeth or use mouthwash on the day of test and avoid doing strenuous physical exercises 24 h before the tests.
Table 1. Baseline characteristics of HIV-infected patients
(Mean values and standard deviations; numbers)

SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LT-CD4, lymphocytes T-CD4; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INI, integrase inhibitor.
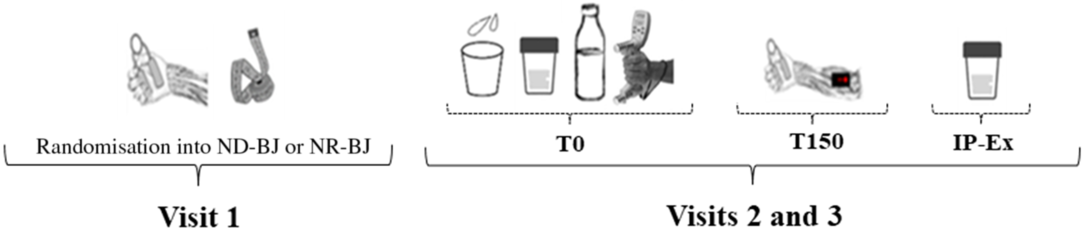
Fig. 2. Schematic overview of experimental measurements. ND-BJ, nitrate-depleted beetroot juice; NR-BJ, nitrate-rich beetroot juice; IP-Ex, immediately after exercise protocol. ![]() , Anthropometric measurement;
, Anthropometric measurement; ![]() , urinary sample;
, urinary sample; ![]() , salivary sample;
, salivary sample; ![]() , ND-BJ or NR-BJ consumption;
, ND-BJ or NR-BJ consumption; ![]() , handgrip exercise protocol and near-IR spectroscopy measures;
, handgrip exercise protocol and near-IR spectroscopy measures; ![]() , handgrip familiarisation;
, handgrip familiarisation; ![]() , maximal voluntary contraction measure.
, maximal voluntary contraction measure.
Beetroot juice preparation
Red beetroots (Beta vulgaris L.) were provided by a supplier located in Macaé, Rio de Janeiro, Brazil. The beetroots were weighed, cleaned with deionised water, and juice was extracted with a centrifugal juicer. The beetroots were processed on the spot and without adding water. The nitrate-depleted beetroot juice (ND-BJ) was prepared according to Baião et al.(Reference Baião, Conte-Junior and Paschoalin15) by using a specific anion exchange resin. The content of nitrate in NR-BJ and ND-BJ consumed by the subjects was 8·12 (sd 3·61) and 0·08 (0·76) mmol/140 ml, respectively. Previous studies have demonstrated vascular benefits after a single dose of dietary nitrate ranging from 5·5 to 22·5 mmol per dose(Reference Webb, Patel and Loukogeorgakis5,Reference Wylie, Kelly and Bailey13,Reference Kapil, Milsom and Okorie16,Reference Oliveira, Morgado and Pierucci17) .
Nitrate and nitrite analysis
Salivary nitrate and nitrite, urinary nitrate and the nitrate content of NR-BJ and ND-BJ were analysed as described by Croitoru(Reference Croitoru18) using HPLC with some modification in sample dilution and solvent elution gradient. Urinary nitrate concentrations were corrected for urinary creatinine concentrations and expressed as mmol/mmol creatinine.
Muscle oxygen saturation measurement
Muscle oxygen saturation (SmO2) and total Hb (tHb) of the flexor muscle (flexor carpi radialis) of each subject’s dominant forearm was continuously monitored using a commercially available, portable NIRS device (PortaMon; Artinis Medical Systems). The software calculates absolute changes in light absorption at different wavelengths (750 and 850 nm) of oxyhaemoglobin (O2Hb) and deoxyhaemoglobin (HHb), as well as tissue O2 saturation index (%TSI) using spatially resolved spectroscopy(Reference Suzuki, Takasaki and Ozaki19). Forearm skinfold thickness of participants was assessed given that the NIRS light could penetrate approximately 18 mm into the tissue, considering an inter-optode distance of 35 mm, as used in the present study. The following NIRS parameters were considered for analysis: baseline SmO2 (SmO2base) was calculated as the average of SmO2 30 s before exercise; muscle O2 desaturation rate (SmO2desat_rate) was calculated as downslope of SmO2 expressed as %/s; SmO2min was considered the minimum SmO2 value achieved during exercise; muscle oxygen resaturation rate (SmO2resat_rate) was calculated as the upslope of SmO2 over 10-s window during exercise recovery; maximal SmO2 (SmO2max) was calculated as the maximum SmO2 value achieved during exercise recovery; total blood volume amplitude (ΔtHbEx) was calculated as the difference between maximum and minimum SmO2 values achieved during exercise; and total blood volume rate (tHbslope) was calculated as the upslope of tHb value over 10-s window during exercise recovery. Handgrip and NIRS parameters have been shown to be valid and highly reliable(Reference Baláš, Kodejška and Krupková20,Reference Celie, Boone and Van Coster21) .
Statistical analysis
An a priori power analysis was conducted (G*Power, version 3.0.1) for a paired t test. On the basis of statistical power (1 – β) of 0·80, an effect size of 0·7 (based on our findings from Oliveira et al.(Reference Oliveira, Morgado and Conte-Junior7) study) and an overall level of significance of 0·05, at least fifteen participants were needed to detect a statistical difference in NIRS parameters (main outcome). To identify differences in SmO2 and tHb parameters, salivary nitrate and nitrite, ETF and MVC between the NR-BJ and ND-BJ interventions, a paired t test was used. To identify differences in urinary nitrate between NR-BJ and ND-BJ, a two-way ANOVA with repeated measures was used. When a significant F was found, additional post hoc tests with Bonferroni adjustment were performed. The homogeneity of variances, normality and sphericity of data were examined with Levene, Shapiro–Wilk and Mauchly tests, respectively. When the assumption of normality and homogeneity of variances was violated, a Wilcoxon (for paired t test) or Friedman (for two-way ANOVA) test was performed to detect a statistical difference. ANOVA was also used to identify a potential sex effect among the participants. Effect size (Cohen’s d) was calculated to observe the magnitude of effect of NR-BJ ingestion compared with ND-BJ, where a value <0·2 was considered trivial, 0·2 to <0·5 was a small effect, 0·5 to <0·8 was a moderate effect and ≥0·8 was a large effect. All analyses were performed using a commercially available statistical package (IBM SPSS Statistics, version 23 for Mac). The results were expressed as means and standard deviations.
Results
Of the twenty-two eligible participants (100 %), fifteen (eleven men and four women) (68 %) completed the study. Among the seven participants who declined to participate (32 %), three withdrew for personal reasons, and four withdrew due to illness unrelated to the study. Among the personal reasons for declining, discomfort of the exercise protocol and duration of each procedure visit (which lasted approximately 4 h each) were most stated by participants. Baseline volunteer characteristics are shown in Table 1.
Nitrate and nitrite analysis
Individual changes in urinary nitrate and salivary nitrate and nitrite concentrations after the NR-BJ and ND-BJ interventions are shown in Fig. 3. There was a significant increase in salivary nitrate (35·78 (sd 21·78) v. 0·28 (0·14) mm, P < 0·001) and nitrite (0·54 (0·26) v. 0·00 (0·00) mm, P < 0·001) after NR-BJ compared with the ND-BJ intervention. Regarding urinary nitrate, there was no significant difference between NR-BJ and ND-BJ at T0 (0·36 (0·03) v. 0·29 (0·03) mmol/mmol creatine, P = 0·207). A significant main effect for time was observed for urinary nitrate (P < 0·001). Post hoc test revealed that urinary nitrate concentration increased more from T0 to IP-Ex after the NR-BJ condition (T0 0·36 (0·03) v. IP-Ex 3·53 (0·37) mmol/mmol creatine, P < 0·001) than after the ND-BJ condition (T0 0·29 (0·03) v. IP-Ex 0·22 (0·37) mmol/mmol creatine, P = 0·869). Furthermore, there was a significant interaction effect (treatment × time) for urinary nitrate (P < 0·001). Post hoc test revealed that urinary nitrate concentration increased at IP-Ex in NR-BJ compared with the ND-BJ condition (3·53 (0·37) v. 0·22 (0·37) mmol/mmol creatine, P < 0·001). There were no significant interaction effects between treatment and sex for salivary nitrate (P = 0·600), nitrite (P = 0·556) and urinary nitrate (P = 0·869). A large effect size was observed for salivary nitrate (d = 1·63) and nitrite (d = 2·07) concentration, as well as for urinary nitrate concentration (d = 2·60). Therefore, the chance of salivary and urinary nitrate concentrations increasing after NR-BJ intake is much higher than ND-BJ intake.

Fig. 3. Individuals’ changes in urinary nitrate (a) and salivary nitrate (b) and nitrite (c) concentrations after the nitrate-rich beetroot juice (NR-BJ) and nitrate-depleted beetroot juice (ND-BJ) interventions. A two-way ANOVA with repeated measures followed by the Bonferroni post hoc test was performed to detect statistical difference in urinary nitrate, and a paired t test was performed to detect statistical difference in salivary nitrate and nitrite. * Significantly different from ND-BJ (P < 0·05).
Muscle oxygen saturation parameters
Table 2 shows the values of all investigated NIRS-derived parameters after the NR-BJ and ND-BJ interventions. Furthermore, individual changes in SmO2min, SmO2max, SmO2desat_rate and SmO2resat_rate after the NR-BJ and ND-BJ interventions are shown in Fig. 4. A representative SmO2 profile (average of fifteen volunteers) during exercise and exercise recovery is shown in Fig. 5. SmO2desat_rate (–7·97 (5·00) v. –5·45 (3·94) %/s, P = 0·005) and SmO2resat_rate (0·43 (0·24) v. 0·28 (0·24) %/s, P = 0·030) were greater after NR-BJ compared with ND-BJ. There were no significant interaction effects between treatment and sex for SmO2min (P = 0·805), SmO2max (P = 0·950), SmO2desat_rate (P = 0·943), SmO2resat_rate (P = 0·921), ΔtHbex (P = 0·644) and tHbslope (P = 0·639). A medium effect size was observed for SmO2desat_rate (d = 0·61), SmO2resat_rate (d = 0·69) and tHbslope (d = 0·45).
Table 2. Changes in muscle oxygen saturation (SmO2) parameters in response to exercise with handgrip after a single dose of nitrate-rich beetroot juice (NR-BJ) and nitrate-depleted beetroot juice (ND-BJ)*
(Means and standard deviations)

SmO2base, SmO2 baseline; SmO2desat_rate, muscle oxygen desaturation rate; SmO2min, minimum SmO2; SmO2resat_rate, muscle oxygen resaturation rate; SmO2max, maximum SmO2; ΔtHbEx, amplitude of blood volume during exercise; tHbslope, blood volume rate.
* A paired t test was performed to detect statistical difference on muscle oxygen saturation parameters. Statistical significance was set at P ≤ 0·05.

Fig. 4. Individuals’ changes in tissue oxygen saturation (SmO2) parameters after the nitrate-rich beetroot juice (NR-BJ) and nitrate-depleted beetroot juice (ND-BJ) interventions. A paired t test was performed to detect statistical difference in muscle oxygen saturation parameters. SmO2desat_rate, muscle oxygen desaturation rate; SmO2resat_rate, muscle oxygen resaturation rate; SmO2min, minimum SmO2; SmO2max, maximum SmO2. * Significantly different from ND-BJ (P < 0·05).

Fig. 5. Representative near-IR spectroscopy profile parameters of tissue oxygen saturation (SmO2) in response to a handgrip exercise protocol after a single dose of nitrate-rich beetroot juice (NR-BJ, ![]() ) and nitrate-depleted beetroot juice (ND-BJ,
) and nitrate-depleted beetroot juice (ND-BJ, ![]() ). SmO2desat_rate (%/s), muscle oxygen desaturation rate; SmO2min, minimum SmO2; SmO2resat_rate (%/s), muscle oxygen resaturation rate (10 s window); SmO2max, maximum SmO2; IP-Ex, immediately after exercise protocol.
). SmO2desat_rate (%/s), muscle oxygen desaturation rate; SmO2min, minimum SmO2; SmO2resat_rate (%/s), muscle oxygen resaturation rate (10 s window); SmO2max, maximum SmO2; IP-Ex, immediately after exercise protocol.
Forearm muscle performance
There was no significant difference in baseline measurement of MVC (362·65 (118·25) v. 364·94 (124·38) N, P = 0·709). Furthermore, no significant difference between the NR-BJ and ND-BJ interventions was observed for ETF (25·23 (3·79) v. 23·63 (7·09) sec, P = 0·390). There was no significant interaction effect between treatment and sex (P = 0·322). A small effect size was observed for ETF (d = 0·28).
Discussion
The major finding of this study was that a single dose of NR-BJ improved the rate of muscle oxygen desaturation and resaturation in HIV-infected patients in response to handgrip exercise. Furthermore, the oral bioconversion of nitrate to nitrite did not seem to be impaired in this population. These results were associated with an increased urinary nitrate excretion.
In the present study, HIV-infected patients exhibited an increased capacity for oxygen extraction at the skeletal muscle level after a single dose of beetroot juice, as shown by the faster downslope of oxygen saturation during exercise, suggesting improvements in oxidative capacity and mitochondrial function. Our findings are in agreement with a previous study showing increased oxygen extraction during exercise after dietary nitrate consumption in older adults with the presence of risk factors for CVD(Reference Oliveira, Morgado and Conte-Junior7). Although different populations have been herein compared, changes in metabolic and mitochondrial functions have been reported in both HIV-infected patients and older individuals with risk factors for CVD(Reference White22,Reference Haas23) .
Higher oxidative capacity is important for a successful performance of activities of daily living and occupational activities, since the ability to sustain submaximal exercise is largely dependent on the oxidative capacity of mitochondria within the skeletal muscle. Therefore, although no statistical difference between NR-BJ and ND-BJ was observed for ETF in the present study, HIV-infected participants were able to support approximately 10 % more time performing the exercise after NR-BJ compared with the ND-BJ condition (NR-BJ 25·23 (3·79) v. ND-BJ 23·63 (7·09) s). Therefore, this modest 10 % difference in ETF (despite observing a small effect size; d = 0·28) associated with an increased capacity for oxygen extraction observed during exercise may suggest the positive impact of beetroot juice intake and/or a diet containing nitrate-rich vegetables on daily living and occupational activities of HIV-infected patients.
During exercise recovery, faster SmO2resat_rate and blood volume response (tHbslope) were observed after NR-BJ compared with ND-BJ, suggesting improved skeletal muscle microvascular perfusion(Reference Alvares, Oliveira and Soares24). This finding is in agreement with a previous study from our laboratory that demonstrated faster SmO2resat_rate and greater blood volume response during the recovery period of handgrip exercise in older people(Reference Oliveira, Morgado and Conte-Junior7). During a high-intensity exercise, Hb saturation decreases, which may favour the reduction of nitrite to NO(Reference Bailey, Varnham and DiMenna11,Reference Affourtit, Bailey and Jones12) . This is supported by evidence demonstrating a nitrite reductase activity of deoxyhaemoglobin, in which nitrite reacts with ferrous deoxyhaemoglobin and H+ to generate NO and methaemoglobin(Reference Cosby, Partovi and Crawford25). Although an analysis of skeletal muscle nitrite was not performed in the present study in order to confirm nitrite availability for reduction to NO, the muscle oxygen saturation levels observed during exercise may suggest the presence of a nitrite reductase system involving deoxyhaemoglobin, which may partly explain the improved muscle perfusion (i.e. faster SmO2resat_rate and tHbslope) observed in the present study. These findings are particularly interesting since the enhanced muscle oxygen saturation after NR-BJ ingestion may be associated with improvements in endothelial function, which may be evaluated by physiological parameters (i.e. flow-mediated dilation technique) and/or biochemical markers (i.e. asymmetric dimethylarginine, von Willebrand factor, soluble P-selectin, soluble thrombomodulin, soluble vascular endothelial cadherin) at rest (i.e. without exercise interference). Such an analysis is important given that HIV-infected individuals typically exhibit endothelial dysfunction, which is an early stage of a cardiovascular event(Reference Marincowitz, Genis and Goswami26,Reference Strijdom, De Boever and Walzl27) . This finding certainly merits further investigation.
Once in the oral cavity, nitrate is reduced to nitrite by commensal facultative anaerobic bacteria that show nitrate reductase activity. HIV-1 infection has been associated with increased prevalence of oral mucosal infections and dysregulation of oral microbiota(Reference Salas and Chang28), which may compromise the efficiency of oral nitrate bioconversion to nitrite. However, the increased salivary nitrate and nitrite concentrations observed in the present study suggest that nitrate bioconversion to nitrite in the oral cavity was preserved in HIV-infected patients. It is important given that a limiting step for nitrate bioconversion to nitrite is the oral microbiota(Reference Lundberg, Weitzberg and Gladwin29), despite evidence demonstrating that enteric bacteria (i.e. Escherichia coli) encodes nitrate reductase activity, thereby contributing to an increase in the body’s nitrite pool(Reference Tiso and Schechter30). Nitrate that escapes from being entirely reduced to nitrite in the oral cavity is rapidly absorbed by the upper gastrointestinal tract(Reference Lundberg, Weitzberg and Gladwin29). Although approximately 25 % of ingested nitrate is absorbed by the salivary glands, most of the nitrate is excreted in the urine (65–70 %)(Reference Bartholomew and Hill31). The increased urinary nitrate observed in the present study after NR-BJ intake (13·3-fold compared with ND-BJ) suggests that dietary nitrate may have been mixed with the nitrate that formed from the oxidation of endogenous NO produced by NOS enzymes and, hence, excreted into urine.
It is important to point out that one of the limitations of the present study was not evaluating plasma nitrite and nitrate. Even though the assessment of plasma nitrate and nitrite would strengthen the findings of the present study, changes in plasma nitrate and nitrite cannot be exclusively accredited to NO metabolism. Increased plasma nitrate and nitrite may be merely a result of NR-BJ ingestion, oxidation of endogenous NO formed by the NO synthase enzyme, or a mix of both(Reference Lundberg, Weitzberg and Gladwin29). Overall, the importance of circulatory and excretory nitrate and nitrite as a measure of NO synthesis is limited because of the complex and incompletely understood biology of NO, nitrate and nitrite.
Recent evidence suggests that the skeletal muscle may serve as a key site for nitrate storage and metabolism, and it may therefore be a novel potential regulator of NO bioavailability following nitrate intake(Reference Wylie, Park and Vanhatalo32). Nitrate stored in the skeletal muscle may be rapidly converted to nitrite via nitrate reductase system (i.e. xanthine oxidoreductase and perhaps aldehyde oxidase), and hence to NO in situations of increased oxygen consumption (i.e. via nitrite reductase system involving deoxyhaemoglobin), such as during exercise. Therefore, a lack of information about skeletal muscle nitrate and nitrite concentrations is another important limitation of the present study. However, Wylie et al.(Reference Wylie, Park and Vanhatalo32) demonstrated increased skeletal muscle nitrate stores 2 h after NR-BJ intake with a significant reduction following a high-intensity exercise, suggesting the conversion of nitrate stores to nitrite and NO (which was likely exercise-induced). Therefore, the activation of the nitrate–nitrite–NO pathway may likely have been the most plausible mechanism by which NR-BJ ingestion induced improvements in muscle oxygenation parameters in response to exercise in HIV-infected individuals(Reference Bailey, Varnham and DiMenna11,Reference Affourtit, Bailey and Jones12) .
Of the fifteen participants in this study, it is noteworthy that four were female. Although there is evidence demonstrating that females have higher circulating levels of nitrite compared with males(Reference Kapil, Rathod and Khambata33), no significant sex effect was detected in the present study.
Conclusion
The results of the present study suggest that dietary nitrate improves muscle oxygen saturation levels in HIV-infected patients by improving the rate of muscle oxygen desaturation and resaturation in response to a handgrip exercise. Furthermore, beetroot juice intake increased urinary nitrate excretion and promoted oral bioconversion of nitrate into nitrite. Our findings suggest that beetroot juice intake may acutely improve muscle oxygen saturation in HIV-infected patients undergoing HAART. Future studies investigating the chronic effects of dietary nitrate intake on endothelial function, as well as measurements of skeletal muscle nitrate and nitrite may be warranted to provide a better nutritional strategy based on nitrate-rich foods for people living with HIV-1.
Acknowledgements
The authors would like to thank Ricky Toledano for the preparation of the English version of the manuscript.
This work was supported by the Fundação Carlos Chagas de Amparo a Pesquisa do Estado do Rio de Janeiro – FAPERJ (E-26/010.100981/2018 and E-26/202.905/2019).
Formulating the research question(s): T. S. A. and E. B.-S. Designing the study: T. S. A., E. B.-S. and G. V. O. Carrying it out: E. B.-S., G. V. O. and M. V.-S. Analysing the data: E. B.-S. and G. V. O. Writing the article: E. B.-S., G. V. O., M. V.-S. and T. S. A.
The authors declare that there are no conflicts of interest.












