Dietary fibres are food components that have essential effects in digestion in humans and animals. Fibre affects large-bowel function, causing an increase in stool output, dilution of colonic contents, a faster rate of passage through the gut and changes in the colonic metabolism of minerals, N and bile acids(Reference Stephen and Cummings1). Soluble dietary fibre, such as pectin, is not digested by endogenous enzymes but fermented in the large intestine. Colonic fermentation leads to the production of SCFA, which are used as an energy source by enterocytes. Both the high fermentation rate and high viscosity induced by soluble dietary fibre supplementation may be responsible for the observed stimulation of intestinal mucosal cell proliferation(Reference Gee, Lee-Finglas, Wortley and Johnson2).
Indeed pectin has been reported to have a trophic effect on the pancreas and intestine(Reference Bamba, Sasaki and Hosoda3), to stimulate intestinal cell proliferation and to increase brush-border membrane enzyme activities(Reference Chun, Bamba and Hosoda4). It was also demonstrated that pectin supplementation increased the levels of plasma enteroglucagon (peptides derived from proglucagon processing by intestinal L cells) in rats(Reference Chun, Bamba and Hosoda4), which is a humoral factor for intestinal mucosal growth(Reference Sasaki, Fitzgerald, Mandir, Sasaki, Wright and Goodlad5). In addition, dietary pectin has been shown to modulate immune function in the intestine(Reference Lim, Yamada, Nonaka, Kuramoto, Hung and Sugano6, Reference Yamada, Tokunaga, Ikeda, Ohkura, Mamiya, Kaku, Sugano and Tachibana7) and spleen(Reference Yamada, Tokunaga, Ikeda, Ohkura, Mamiya, Kaku, Sugano and Tachibana7), and also the hepatic metabolism of cholesterol(Reference Hexberg, Hexeberg, Willumsen and Berge8) and biliary acids(Reference Ide and Horii9). This may have consequences for protein metabolism in these organs, but to our knowledge no data have been published about the effect of pectin on protein synthesis rate in intestinal tissues, except in our previous study(Reference Pirman, Ribeyre, Mosoni, Rémond, Vrecl, Salobir and Patureau Mirand10), or in other splanchnic or peripheral organs. Our hypothesis was that stimulation of protein metabolism in the intestines by pectin feeding could have consequences on protein metabolism in other organs.
Hence the present study was designed to evaluate the effect of citrus pectin on protein metabolism in the intestinal tissues, visceral organs and in skeletal muscles of growing rats, which were chosen as the animal model.
Material and methods
Diets
Two diets (Table 1) were prepared, the control diet and the 8 % pectin diet in which a fraction of the wheat starch was replaced with pectin from citrus peel (Fluka reference no. 76280; degree of esterification 63–66 %; molecular weight 30 000–100 000 Da). The diets contained an oil mixture adjusted so that both diets were isoenergetic (15·3 kJ/g metabolisable energy). The protein source of the diets was casein, purchased from Union des Caséineries de Charente Maritime (La Rochelle, France) and diets contained 110 g crude protein per kg. Diets were designed to meet the nutritional requirements of growing rats.
Table 1 Diets
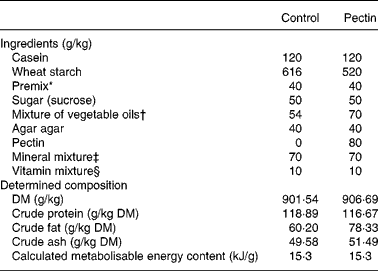
* Mixture of wheat starch and l-cystine 55 mg/g (2·2 g cystine added in each diet).
† Rapeseed oil, groundnut oil and sunflower-seed oil (50:45:5, by vol.).
‡ UAR 205 b (UAR, Villemoisson-sur-Orge, France).
§ UAR 2.00.
Animals
All procedures were in accordance with the guidelines formulated by the European Community for the use of experimental animals (L358-86/609/EEC). Twenty-four male weaning Sprague–Dawley rats (60·96 ± 2·68 g body mass) from Charles River (Lyon, France) were housed in a ventilated room at a controlled temperature (21°C) with a 12 h light–dark cycle (starting at 04.00 hours) and placed in individual cages with free access to drinking water. In the adaptation period (10 d), the rats received commercial pellets ad libitum. After the adaptation period rats were separated into two identical groups; one received the control diet and the other the pectin diet. The experimental period lasted 14 d. During the first week animals were pair-fed to avoid large differences in food intake induced by the food change with the pectin diet. They were fed ad libitum during the second week during which growth performance measurements were performed.
Tissue sampling
At the end of the experimental period, in vivo tissue protein synthesis rates were determined 17–20 h after the last daily food distribution by the flooding dose method(Reference Garlick, McNurlan and Preedy11) using l-[13C]valine (150 μmol/kg, 50 % enrichment; Euriso-Top, Saint Aubin, France). For each group, the basal enrichment of protein-bound valine in the tissues was determined in two rats. A flooding dose of [13C]valine was intravenously injected into the tail vein of the other ten rats of each group. At 15 min after [13C]valine injection the rats were anaesthetised by intraperitoneal injection of 0·3 ml sodium pentobarbital. The abdominal wall was opened and rats were exsanguinated by sampling from the abdominal aorta with an EDTA-coated syringe 20 min after valine injection. Liver, spleen, gastrocnemius muscle, small intestine, caecum and colon were quickly excised and chilled on ice to stop tracer incorporation.
Stomach, small intestine and large intestine were quickly weighed with their contents, emptied, rinsed with a cold 2 % TCA solution, wiped, put in labelled pre-weighed bags, weighed and frozen as soon as possible in liquid N2.
Simultaneously liver, spleen and gastrocnemius muscle from the right hind leg of the animals were quickly dissected. All samples were weighed, frozen in liquid N2 and kept at − 80°C until further analysis. The empty body mass was calculated from the difference between the body mass and the masses of the contents of the stomach, small and large intestine. As digestive contents have been reported to be increased by pectin feeding, this allowed us to calculate organ relative mass (g/100 g empty body mass) in order to detect changes in body composition more accurately.
Protein metabolism measurements and analytical methods
Protein synthesis activity was assessed by the absolute synthesis rate (ASR; mg/d) and translational efficiency, calculated as the amount of protein synthesised (mg)/d per mg RNA:
where t is the incorporation time, EP is the 13C enrichment of protein-bound valine at the time of death in the injected rats, EN is the natural enrichment of protein-bound valine, EA is the enrichment of tissue-free valine at the time of death in the injected rats and prot is the total tissue protein content. The [13C]valine enrichment in the free amino acid pool and in the protein pool of the tissues was measured as described elsewhere(Reference Arnal, Mosoni, Dardevet, Ribeyre, Bayle, Prugnaud and Patureau Mirand12), together with determination of the protein contents(Reference Smith, Kroh, Hermanson, Mallia, Gartner, Provenzano, Fujimoto, Goeke, Olson and Klenk13), RNA contents(Reference Manchester and Harris14) and DNA contents(Reference Giles and Myers15). Briefly, free valine enrichment determination was performed on 0·6 m-TCA extracts by GC-MS, with an HP 5972 organic mass spectrometer quadrupole coupled to an HP 5890 GC (Hewlett Packard, Les Ulis, France). Valine was measured as the tertiary butyl-dimethlsilyl derivative under electron impact ionisation. The ions m/z 288 and 289 were monitored by selective ion recording to determine the [13C]valine enrichment. For determination of the 13C enrichment of protein-bound valine, tissue protein precipitates were hydrolysed in 6 m-HCl. Valine was measured as N-acetyl-propyl-amino acid as derivative. The 13CO2:12CO2 ratio was determined with GC-combustion isotope ratio MS (Isochrom; Fisons, Manchester, UK). Blood cell counts (leucocytes and erythrocytes) were determined with an automatic haematology counter (Minos; ABX, Montpellier, France). Myeloperoxidase (MPO) activity as a marker of neutrophil infiltration in tissues was assayed in the ileum according to the method of Krawisz et al. (Reference Krawisz, Sharon and Stenson16).
Statistical methods
Results are presented as mean values and standard deviations. The number of rats was twelve per group except in tables with protein synthesis data because two rats per group were used to determine 13C natural enrichment of protein-bound valine. The effect of the pectin diet was analysed using Student's t test or the non-parametric Mann–Whitney test if variances were different (SAS Institute, Inc., Cary, NC, USA)(17).
Results
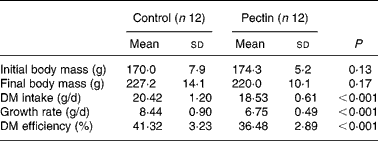
The effect of pectin on growth performance is given in Table 2. DM intake, growth rate and DM efficiency (g body-weight gain/g DM intake) were significantly lower in the pectin group than in the control group. No difference was detected in blood cell counts (not shown), except a significant increase (P = 0·007) in the number of lymphocytes (3·4 (sd 0·4) v. 4·2 (sd 0·6) × 103 per mm3 in the control and pectin rats, respectively).
Table 2 Effect of pectin feeding on animal body mass, dry matter intake and growth rate
(Mean values and standard deviations)
The effect of pectin feeding on organ mass is shown in Table 3. Supplementation of the diet with pectin significantly decreased the mass of gastrocnemius muscle and spleen (P < 0·05) and, to a lesser extent, of stomach (P = 0·053); nevertheless this corresponded to the decrease in body mass, since if these values are expressed as g/100 g empty body mass the differences disappear. The mass of the liver was significantly decreased in the pectin group, irrespective of the mode of presentation of the results (g or g/100 g empty body mass). Intestinal tissues, small intestine and large intestine were significantly heavier in the pectin group. Differences were more pronounced in the small intestine (+73 %) than in the large intestine (+34 %). Similar differences were found when the values were expressed as g/100 g empty body mass. In the ileum, MPO activity was low but significantly higher (P = 0·002) in the pectin-fed rats than in the control rats (1·38 (sd 0·73) v. 0·42 (sd 0·16) U/g protein).
Table 3 Effect of pectin feeding on empty body and organ masses
(Mean values and standard deviations)
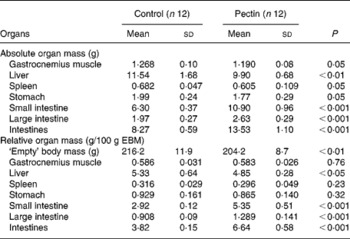
EBM, empty body mass (body mass without intestinal content).
In gastrocnemius muscle, the absolute amount of protein and RNA was decreased (Table 4), as was its mass. The values for absolute protein synthesis rate (mg protein synthesised in the organ/d) and translational efficiency, which represents the mass of mg protein synthesised/d per mg RNA, were significantly decreased in the pectin group as compared with the control group. In the pectin group, they reached only 81 and 86 % of the values in the control group, respectively. Fractional protein synthesis rates were similarly lower in the pectin group than in the control group (9·86 (sd 1·13) and 11·32 (sd 0·79); P = 0·007).
Table 4 Effect of pectin feeding on protein metabolism in gastrocnemius muscle
(Mean values and standard deviations)
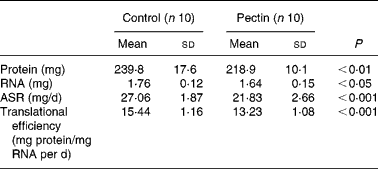
ASR, absolute synthesis rate.
Although the liver mass was lower in the pectin group than in the control group, the absolute amounts of protein and protein synthesis rates were not different in the two groups. However, the fractional protein synthesis was significantly higher (P = 0·011) in the pectin group than in the control group (71·31 (sd 7·29) and 58·45 (sd 11·28) %/d, respectively). As RNA content was significantly decreased by pectin feeding ( − 12 %) (Table 5), translational efficiency was significantly increased (+32 %). The effects of pectin feeding in the spleen were similar to those in the liver but to a lesser extent, and only the decrease in RNA content ( − 23 %) was significant.
Table 5 Effect of pectin feeding on protein metabolism in liver and spleen
(Mean values and standard deviations)
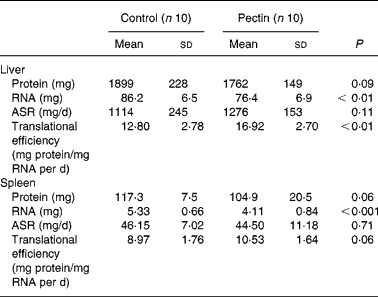
ASR, absolute synthesis rate.
The largest effect of pectin feeding was found in intestinal tissues (Table 6), where all values were significantly increased by pectin feeding and to a similar extent in the small and large intestine. The absolute protein and RNA amounts, translational efficiency and absolute protein synthesis rates were increased by 54 and 57, 68 and 59, 32 and 35, 114 and 115 %, respectively in the small and large intestine. Consequently, the same effects were observed for the whole intestine.
Table 6 Effect of pectin feeding on protein metabolism in intestinal tissues
(Mean values and standard deviations)
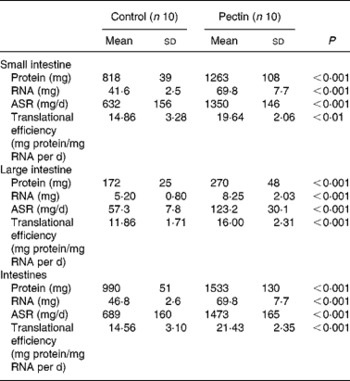
ASR, absolute synthesis rate.
Discussion
The present study confirms the trophic effect of pectin, a soluble dietary fibre, on intestinal tissues. Protein metabolism in intestinal tissues and in the liver was significantly stimulated by pectin feeding, but, on the other hand, protein metabolism in gastrocnemius muscle was reduced. The effect of pectin in the spleen was low.
As expected, the most striking effect of pectin feeding was detected in the intestinal tissues, where the organ masses and their protein and RNA amounts were increased. Similar hypertrophy of gut tissues after consuming fibrous diets has been reported in rat(Reference Goodlad and Mathers18–Reference Zhao, Jørgensen and Eggum20) and pig(Reference Jørgensen, Zhao and Eggum21) studies. Fukunaga et al. (Reference Fukunaga, Sasaki, Araki, Okamoto, Yasuoka, Tsujikawa, Fujiyama and Bamba22) have already demonstrated that pectin has a significant trophic effect on the ileum, caecum and colon in rats fed a diet supplemented with 2·5 % pectin. In the present study, the hypertrophies in intestinal tissues were well marked in the small and large intestine. This corresponds to a stimulation of protein synthesis in all parts of the intestines. This is consistent with an increase of intestinal endogenous protein secretion and enterocyte renewal(Reference Fairweather-Tait, Gee and Johnson23). It could be attributed to a stimulated microflora activity. Bacterial fermentation of dietary fibre in the caecum and colon produces SCFA which in turn induce cell proliferation in the lower intestinal tract. Our previous results(Reference Pirman, Ribeyre, Mosoni, Rémond, Vrecl, Salobir and Patureau Mirand10) showed that the same pectin diet stimulated the production of caecal and especially colonic SCFA, namely acetic, propionic and butyric acids. SCFA are considered to be the major mediators that stimulate intestinal mucosal growth(Reference Sakata and Yajima24, Reference Johnson, Gee and Brown25). SCFA were also shown to increase glucagon-like peptide 2 secretion by intestinal tissues(Reference Burrin, Guan, Stoll, Petersen and Sangild26), which is a potent growth factor for intestinal mucosa. This effect on glucagon-like peptide 2 secretion was already proven with pectin(Reference Fukunaga, Sasaki, Araki, Okamoto, Yasuoka, Tsujikawa, Fujiyama and Bamba22) and some other dietary fibres(Reference Reimer, Thomson, Rajotte, Basu, Ooraikul and McBurney27, Reference Nian, Gu, Irwin and Drucker28).
The stimulation of protein metabolism by pectin feeding in the liver (translational efficiency and fractional protein synthesis rates) had no effect on liver protein weight, suggesting either an increase in protein degradation and/or in protein exportation because most of the plasma proteins are synthesised in the liver. As the intestine is a major site for breakdown and clearance of plasma protein, it is likely that the increase in intestinal metabolism caused an increase in plasma protein degradation which could be compensated for by stimulation of their synthesis in the liver. Consistent with this assumption, a decrease in serum albumin levels was reported in rats fed a low-protein diet when pectin was added(Reference Rotenberg and Jakobsen29). Whether an increase in cholesterol synthesis(Reference Hexberg, Hexeberg, Willumsen and Berge8) and excretion of biliary salts in pectin-fed animals(Reference Ide and Horii9) could also contribute to the observed increase in liver protein metabolism is not known.
A marked stimulation of both intestinal and hepatic protein synthesis has also been described in acute inflammation(Reference Breuillé, Arnal, Rambourdin, Bayle, Levieux and Obled30). Because the effect of pectin feeding on protein synthesis in the spleen was only a slight increase in translational efficiency, this indicates that it did not induce an acute inflammation response. However, there were some parameters that could indicate a slight stimulation of the immune response: the tendency to an increase of translational efficiency in the spleen, the increase in lymphocyte number in the blood, and in MPO activity in the ileum. These results are consistent with data in the literature showing an increase in IgA in serum, and IgA and IgG in the spleen and mesenteric lymph node lymphocytes(Reference Lim, Yamada, Nonaka, Kuramoto, Hung and Sugano6, Reference Yamada, Tokunaga, Ikeda, Ohkura, Mamiya, Kaku, Sugano and Tachibana7). However, the slight difference in MPO activity could also result from a difference in total peroxidase activity induced by the increase in whole-intestine metabolic activity, since the method used is not strictly specific for MPO(Reference Krawisz, Sharon and Stenson16).
In our earlier studies with legumes added in the diet, protein metabolism in gastrocnemius muscle was decreased, if there was an effect on body-weight gain(Reference Pirman, Combe, Ribeyre, Prugnaud, Stekar and Patureau Mirand31) and was almost unchanged if there was no difference in the body-weight gain(Reference Pirman, Combe, Patureau Mirand, Stekar and Orešnik32, Reference Combe, Pirman, Stekar, Houlier and Patureau Mirand33), which is in accordance with results of the present study. Gastrocnemius muscle is sensitive to the nature of the nutrients derived from the diet(Reference Frühbeck, Villaro, Monreal and Santidrian34) and, as a fast-twitch oxidative glycolytic muscle, is a good indicator of the average response of whole skeletal muscle(Reference Waterlow, Garlick and Millward35). The lowering effect of pectin feeding on muscle protein synthesis is not likely to be induced by a difference in last food intake because protein synthesis was determined 6 h after the beginning of the light period, i.e. when rats were not active and 18 h after the time at which food was offered. Furthermore, last food intake was not different in the two groups (20·6 (sd 3·3) and 20·2 (sd 2·1) g DM in the control and the pectin groups, respectively). In addition, the low and equal amount of food left in the stomach at slaughter (8·3 (sd 4·5) and 9·2 (sd 1·7) % of the ingested amounts for the pectin-fed and control rats, respectively) indicates that the amount of nutrients submitted to intestinal digestion was not different at that time. However, N faecal losses, mainly of endogenous origin, are known to be increased in pectin-enriched diets(Reference Pastuzewska, Kowalczyk and Ochtabinska36, Reference Mosenthin, Sauer and Ahrens37), resulting in an increased amino acid loss. Despite similar intakes of nutrients, there may be differential nutrient availability at peripheral levels due to large uptakes at splanchnic levels because of protein synthesis stimulation and due to increased endogenous losses. This may help to explain the present effect of pectin on muscle protein synthesis. Low-protein diets can enhance such an effect. This increase in the alteration of food efficiency and protein efficiency ratio by pectins has already been shown with low-protein diets(Reference Samman, Medici, Rossi and Farias38). It may suggest an increase in specific or global amino acid requirements of young growing rats during this 2-week period that could be considered as an adaptation period, as suggested by experiments in pigs fed wheat shorts-containing diets(Reference Libao-Mercado, Yin, van Eys and de Lange39).
Pectin feeding resulted in a specific pattern of protein metabolism activities in organs characterised by an increase in protein turnover, i.e. in intestines and in the liver, and a decrease in muscle. If there are increasing data that could explain the causes of these alterations in the intestines, these factors are still unknown for the other organs. Further studies are needed to determine what could be the impact of the probable stimulation of the immune function, because it was shown that muscle protein anabolism was lessened in the case of low-grade inflammation(Reference Mercier, Breuillé, Mosoni, Obled and Patureau Mirand40). These modifications are considered to affect the partitioning of nutrients between organs. Stimulation of protein synthesis in splanchnic tissues could reduce nutrient availability in peripheral organs. Additional investigations are needed to discover whether this partitioning is implicated in the alteration of the between-organ metabolic pattern induced by pectin feeding.
Acknowledgements
The authors are grateful to Christian Lafarge for animal care and Anne Diot and Corine Pouyet for participation in tissue protein synthesis determination. The authors had no conflicts of interest. This research was supported by the Proteus Bilateral Programme between Slovenia (Slovenian Research Agency) and France (French Research Ministry).
The contribution of each author was as follows: T. P., conception and design, tissue sampling, data processing, drafting and revision of the manuscript; L. M. and D. R., conception and design, tissue sampling, revision of manuscript; M. C. R., protein synthesis rate determinations; C. B., design, tissue sampling, blood cell counts and MPO determinations, data processing; J. S., conception and design, tissue sampling, data processing, revision of manuscript; P. P. M., conception and design, animal experiment, tissue sampling and revision of manuscript.










