Amongst biotechnological additives, feed enzymes (FE) for swine and poultry have made the most progress and impact in the past decade. Generally, the enzyme systems available for the animal feed industry are derived from microbes (fungi and bacteria) through traditional submerged liquid fermentation(Reference Bailey and Olis1) or solid-state fermentation(Reference Mitchell, Lonsane, Doelle, Mitchell and Rolz2). Recent estimates indicate that FE saved the global feed market an estimated US $3–5 billion per year(Reference Adeola and Cowieson3). This value emanated from considerable investment in application research leading to strategic developments in the use of FE and, as a result, the creation of a global FE market worth in excess of US $650 million and growing(Reference Adeola and Cowieson3, Reference Barletta, Bedford and Partridge4). Phytase currently dominates this market, taking 60 % share, carbohydrase 30 % and the rest (proteases, lipases, etc.) 10 %(Reference Adeola and Cowieson3). Rapid growth in the last decade has been associated with the acceptance of phytase in replacing inorganic phosphates and the wider use of carbohydrases in maize-based diets to mitigate spiralling feed costs and minimise nutrient excretion. The carbohydrase market is accounted for by two dominant enzymes: xylanases and cellulases. Other commercially available carbohydrases include α-amylase, β-mannanase, α-galactosidase and pectinase(Reference Adeola and Cowieson3).
The role of FE in improving the productive value of diets for single-stomached animals has received extensive reviews, for example, Bedford & Schulze(Reference Bedford and Schulze5), Adeola & Cowieson(Reference Adeola and Cowieson3), Woyengo & Nyachoti(Reference Woyengo and Nyachoti6) and Slominski(Reference Slominski7). In this context, several modes of action have been proposed, namely: (1) hydrolysis of specific chemical bonds in feedstuffs that are not sufficiently degraded or indeed not at all by the animal's own enzymes (for example, mixed salts of phytic acid); (2) elimination of the nutrient-encapsulating effect of the cell wall polysaccharides and therefore increased availability of starches, amino acids and minerals; (3) breakdown of anti-nutritional factors that are present in many feed ingredients (for example, soluble NSP and phytic acid); (4) solubilisation of insoluble NSP for more effective hindgut fermentation and thus improved overall energy utilisation; and (5) complementation of the enzymes (for example, amylase, protease, lipase) produced by young animals where, because of the immaturity of their own digestive system, endogenous enzyme production may be inadequate.
Recent price volatility of traditional feed ingredients suggests that the swine and poultry industries will continue to seek alternative cost-effective feed ingredients such as cereal co-products from biofuel and milling industries(Reference Kiarie and Nyachoti8). However, the successful application of alternative ingredients will be dependent on the characterisation of their nutritive value, availability of technologies for mitigating risks associated with them (for example, mycotoxins, anti-nutritional factors) and potential economic benefits when formulated correctly into swine and poultry diets. As an example, the high NSP and indigestible protein contents in cereal co-products can limit their inclusion into pig feed(Reference Bach Knudsen9); however, supplemental NSP-degrading enzymes and proteases might allow high inclusion of such feedstuffs. The ability to find and evolve the next generation of FE will be driven by understanding the target substrates and the implications to animal nutrition(Reference Bedford, Partridge, Bedford and Partridge10). However, the gastrointestinal tract (GIT) is populated with diverse assemblages of microbiota that play a critical role not only for the overall well-being of the animal, but also for its nutrition, performance and quality of its products. In this context, there is a clear need to understand the role of FE in influencing gut health through modulation of the gastrointestinal microbiota. The present review is an attempt to summarise current thinking in this area, underscore mechanisms, and suggest opportunities for expanded exploitation of FE biotechnology.
Swine and poultry gut microbiomes
A complex and dense community of bacteria, archaea, fungi, protozoa and viruses inhabits the gut of the pig and the chicken. Indeed, the total number of microbial cells within the GIT of single-stomached animals, including man, exceeds that of the host cells by at least one order of magnitude(Reference Savage11). The GIT microbiome exhibits a gradient concentration, with numbers and diversity increasing from proximal to distal. For example, in pigs, the stomach and proximal small intestine contain relatively low numbers of bacteria (103–105 bacteria/g or ml of contents); with dominant bacteria being Lactobacillus spp. and Streptococcus spp.(Reference Jensen12). In contrast, the distal small intestine harbours a more diverse and numerically greater (108 bacteria/g or ml of contents) population of bacteria(Reference Gaskins, Lewis and Southern13). In broiler chickens, analysis of 16S rDNA gene sequences revealed thirteen, eleven, fourteen, twelve, nine and fifty-one operational taxonomic units in the crop, gizzard, duodenum, jejunum, ileum and caecum, respectively(Reference Gong, Si and Forster14). Radial distribution of microbes within specific GIT segment has also been described(Reference Gaskins, Lewis and Southern13). The four micro-habitats include: the intestinal lumen; the unstirred mucus layer or layer that covers the mucosal epithelium; the deep mucus layer found in the crypts; and the surface of the intestinal epithelial cells. The diversity of bacterial populations within a particular micro-habitat in the GIT is influenced by factors such as digesta flow rate, pH, anoxic conditions, types of endogenous and dietary substrates, inhibitory factors such as bacteriocins and SCFA, and competitive advantage(Reference Gaskins, Lewis and Southern13, Reference Pluske, Pethick and Hopwood15). Given this scenario, it is likely that certain nutrients and their associated physico-chemical effects play a major role in maintaining the balance of the microflora in specific micro-habitats, and subsequently in determining whether a pathogenic bacterium proliferates.
A significant hindrance in studying the gut microbiome has been the inability to effectively identify and quantify microbial species, their metabolic endproducts, and mechanisms by which they affect host health(Reference Zoetendal, Collier and Koike16, Reference Torok, Hughes and Mikkelsen17). This is mainly because the bulk of available information is limited to cultivable microbiota. The fraction of micro-organisms that are cultivable remains low mainly because the growth requirements of most of the bacteria are unknown, but also because of the selectivity of the isolation media that are used, the stress imposed on bacteria by the cultivation procedures, the need for anoxic conditions and problems simulating the microbe–microbe and microbe–host interactions that occur naturally in the gut(Reference Zoetendal, Collier and Koike16). Some of these issues have been overcome by the increased use of culture-independent, molecular-based techniques that use the sequence diversity of the 16S rDNA gene to explore the diversity and abundance of bacterial communities within the GIT of animals. The use of 16S rDNA sequencing has been applied to analyse the intestinal bacterial community in a number of species, including swine and poultry, and has shown that the majority of the bacterial species colonising the GIT have not been cultured and characterised(Reference Danzeisen, Kim and Isaacson18, Reference Kim, Borewicz and White19). These data provide crucial information on the community structure in the GIT, but they provide limited information on microbe–microbe and host–microbe interactions(Reference Danzeisen, Kim and Isaacson18). Microbes in the GIT have evolved mechanisms to influence the intestinal environment for their own benefit, and potentially that of the host, by influencing epithelial host cell gene expression(Reference Gaskins, Lewis and Southern13, Reference Zoetendal, Collier and Koike16). Host–microbe interaction is currently a very active area of research and may help in identifying clusters of GIT bacteria that are consistently associated with better growth performance and health in animals raised in varied environments(Reference Torok, Hughes and Mikkelsen17, Reference Stanley, Geier and Hughes20).
Concept of dietary approaches to gastrointestinal health
The primary functions of the GIT are to digest and absorb nutrients from the diet and to excrete waste products. As alluded to, the GIT also contain normal microbiota responsible for a plethora of functions including intestinal development and functionality (as evidenced by differences seen between gnotobiotic and conventional animals), nutrient digestion and absorption, mucus secretion, immune development and cytokine expression(Reference Gaskins, Lewis and Southern13, Reference Klasing21, Reference Niba, Beal and Kudi22). However, there are many specific bacterial pathogens that also inhabit the GIT, and they generally cause disease when the gut ecosystem is disturbed in some manner. For example, in the post-weaning period in pigs, numbers of pathogenic Escherichia coli proliferate to exceed those of other bacterial populations, resulting in clinical disease(Reference Fairbrother, Nadeau and Gyles23). Many factors influence the diversity and activity of the GIT microbiota, including the age of the animal and the environment it inhabits, antimicrobial agents (antibiotics and minerals such as Zn and Cu), dietary composition (for example, type and content of carbohydrates and protein), feed additives (for example, organic acids; FE), feeding methods (fermented liquid feeding), disease load, weaning, season, stress and genetics(Reference Gaskins, Lewis and Southern13, Reference Pluske, Pethick and Hopwood15, Reference Zoetendal, Collier and Koike16, Reference Torok, Hughes and Mikkelsen17). These factors, which usually interact, can make comparison of studies on gut microbiota a daunting task.
One question that generally arises in relation to the GIT microbiome, and particularly the area of gut ‘health’, is: what is ‘normal’ when referring to the health of the pig and chicken gut? Hillman(Reference Hillman24) suggested that emphasis should be placed on an ‘optimal’ GIT microbiota rather than a ‘normal’ microbiota being present, because it is very difficult to define what is ‘normal’ given the wide array of conditions that pigs and chickens are grown under. Producers strive to keep pigs and chickens free of infections (bacteria, viruses, parasites) and achieve the best utilisation of feed for muscle gain and egg production as possible. However, with at least 400 species of bacteria, with numbers as high as 1014 colony-forming units/g inhabiting the GIT(Reference Savage11, Reference Mackie and White25), it is little wonder that perturbations sometimes occur to cause clinical disease and occasionally death(Reference Jensen12, Reference de Lange, Pluske and Gong26). Specific enteric pathogens can cause enormous economic loss to pig and poultry enterprises; hence there is interest in being able to identify, quantify and track the different components of the microbiota (both pathogenic and non-pathogenic) to improve health and production. To check microbial perturbations the industry has long depended on dietary fortification with sub-therapeutic levels of antibiotics and antimicrobial growth promoters (AGP)(Reference de Lange, Pluske and Gong26, Reference Heo, Opapeju and Pluske27). However, long-term use of AGP has been linked to the potential problem of increasing transferable resistance of bacteria to antimicrobial drugs(Reference Adjiri-Awere and Van Lunen28) and has been banned outright in some jurisdictions and increasingly under intense scrutiny in others.
Interest in alternatives to AGP has increased recently as there is a clear need to control enteric pathogens previously contained through the use of AGP in feeds. For example, in poultry there is greater risk of an outbreak of necrotic enteritis (NE) with the use of viscous grains (barley, wheat and rye)(Reference Riddell and Kong29–Reference Jia, Slominski and Bruce31). This has been associated with high digesta viscosity, decreased nutrient digestibility and prolonged intestinal transit time, thus favouring growth of Clostridiumperfringens in the upper gut(Reference Timbermont, Haesebrouck and Ducatelle32). In swine, viscous fibres have also been linked to exacerbation of post-weaning collibacillosis (for example, McDonald et al. (Reference McDonald, Pethick and Pluske33, Reference McDonald, Pethick and Mullan34), Hopwood et al. (Reference Hopwood, Pethick and Pluske35) and Montagne et al. (Reference Montagne, Cavaney and Hampson36)) and swine dysentery (SD) (for example, Durmic et al. (Reference Durmic, Pethick and Pluske37, Reference Durmic, Pethick, Mullan and Cranwell38) and Pluske et al. (Reference Pluske, Siba and Pethick39, Reference Pluske, Durmic and Pethick40)). The detrimental effects of soluble fibres in swine have been associated with increasing digesta viscosity, undigested nutrients in the GIT and endogenous secretions. Furthermore, an increased flow of ileal undigestible protein in the hindgut can result in proteolytic fermentation in the large intestine of pigs and the caecum of poultry that can negatively affect their performance and health(Reference Smulders, Veldman and Enting41, Reference Cone, Jongbloed and Van Gelder42). Arguably, the use of AGP in the past markedly reduced the negative consequences of feeding such feedstuffs and as a result performance was maintained on diets that otherwise would be problematic. Indeed, earlier studies demonstrated that the growth-depressing effect of viscous rye was ameliorated by antibiotic supplementation(Reference Marquardt, Ward and Misir43, Reference Antoniou and Marquardt44). Furthermore, Smulders et al. (Reference Smulders, Veldman and Enting41) showed that antibiotics were more effective in diets with low digestible protein content v. in diets with high digestible protein content. Consequently, there is considerable interest in identifying alternative nutritional strategies for managing the microbiota of pigs and chickens when fed antibiotic-free diets.
Influence of feed enzymes on the gastrointestinal microbiota
Enzymes are biological catalysts that speed up reactions and act on specific substrates or reactants. Arguably, characteristics of FE are designed and set by the producing organism given its ecology (substrate). However, the efficacy in animal feed applications depends very much on a completely different set of criteria which are based on the mechanism of action in animal nutrition(Reference Bedford and Schulze5). The link between FE and the GIT microbiome can perhaps be better understood from two points of view (Fig. 1). On one hand are the effects of substrates by themselves on the digesta biochemical characteristics and GIT physiology and on the other hand is the modification of these effects by FE to the extent that the substrates are degraded or modified in the GIT. This view is important because the rationale for the development and application of FE is to target certain dietary substrates (such as phytate and NSP) that are not degraded sufficiently or indeed not at all by the endogenous digestive enzyme array in the GIT. For example, an accepted paradigm in broiler chickens is that increased intestinal viscosity due to soluble NSP is the most important mechanism for poor growth and feed utilisation(Reference Adeola and Cowieson3, Reference Bedford and Schulze5, Reference Choct, Hughes and Wang45). However, the viscous nature of soluble NSP is a digesta biochemical characteristic that is accompanied by or precipitates many small intestine physiological responses such as increased digesta transit times(Reference Almirall, Francesch and Perez-Vendrell46), intestinal mass and turnover rates of mucosa cells(Reference Gee, Lee-Finglas and Wortley47–Reference Daenicke, Bottcher and Jeroch49), mucin and carbohydrate expression of goblet cell glycoconjugates(Reference Fernandez, Sharma and Hinton50) and undigested constituents(Reference Choct, Hughes and Wang45, Reference Jørgensen, Zhao and Eggum51). These responses have been shown to increase small intestine microbial activity, composition and size(Reference Choct, Hughes and Wang45, Reference Hübener, Vahjen and Simon52). Conversely, supplemental NSP-degrading carbohydrases (NSPases, such as xylanase and β-glucanase) reduce digesta viscosity by partial or complete hydrolysis of soluble NSP, improving animal performance and nutrient utilisation but also effecting changes to the composition and metabolic potential of bacterial populations(Reference Choct, Hughes and Wang45, Reference Hübener, Vahjen and Simon52, Reference Torok, Ophel-Keller and Loo53).

Fig. 1 Link between feed enzymes and gut microbiota in poultry and swine. NSPases, NSP-degrading carbohydrases. (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr)
The literature evidence suggests that diet composition is a strong modulator of the composition of the gut microbiota. For example, the relative contributions of host genetics and diet in shaping the gut microbiota was investigated using wild-type mice and ApoA-I knockout counterparts (genetically modified for impaired glucose tolerance) fed different diets using DNA fingerprinting and bar-coded pyrosequencing of 16S rRNA genes(Reference Zhang, Zhang and Wang54). These investigations revealed that 57 and 12 % variation of gut microbiota in mice was accounted for by the diet and genetic background, respectively, suggesting the primacy of diet in determining the composition of the gut microbiota. Within the context of interaction between FE and GIT microbiota, the view (Fig. 1) that FE act on specific components of feed ingredients in most cases explains the role of FE in modulating the gut microbiota. However, it is also plausible that some enzymes such as alkaline phosphatase can act directly on the microbiota by dephosphorylating the outer membrane(Reference Chen, Malo and Moss55, Reference Lallés56), but as far as we know alkaline phosphatases are not a major FE and there is a dearth of data demonstrating their effects on gut microbiota. In view of the foregoing, it has been suggested that FE might influence the intestinal microbiota through two main mechanisms: reducing the undigested substrates; and creating (in situ) short-chain oligosaccharides from cell wall NSP with potential prebiotic effects. These two mechanisms and the more recent findings will now be discussed.
Reducing undigested substrates
The diversity of the microbiome in a gut section reflects in part the types of nutrient substrates in those sections. Bacteria in the GIT derive most of their carbon and energy from luminal compounds (dietary and/or endogenous) which are either resistant to attack by digestive fluids or absorbed so slowly by the host that bacteria can successfully compete for them. Indeed, it has been suggested that the performance improvement attributes of AGP are due to factors such as reduced competition for nutrients in the small intestine, reduced local inflammation due to control of pathogens, and reduced size of intestine(Reference Visek57, Reference Niewold58). Since bacterial species differ in their substrate preferences and growth requirements, the chemical composition and structure of the digesta largely determine the species distribution of the bacterial community in the GIT. Consequently, bacterial community structure and metabolic function are very much dependent upon digesta biochemical conditions, as a result of feed composition and attendant host physiological responses such as endogenous secretions. For example, Apajalahti et al. (Reference Apajalahti, Kettunnen and Graham59) analysed bacteria chromosomal DNA content of guanine and cytosine (G+C) in caecal digesta samples from broiler chickens fed either maize- or wheat-based diets. Compared with wheat, maize favoured low %G+C microbes (20–34 %) at the expense of the higher %G+C bacteria (55–59 %). In swine, Drew et al. (Reference Drew, Van Kessel and Estrada60) reported changes in gut bacterial populations in pigs fed diets containing maize, barley or wheat as the main carbohydrate source, and correlated some changes with the fibre content of the diets. From the foregoing, it is clear that bacterial species differ in their substrate preferences and growth requirements; the chemical composition and structure of the digesta largely determine the species distribution of the bacterial community in the GIT. Consequently, it is important to understand that FE will mediate or modulate microbial status preset by the main dietary ingredients (target substrates).
When digestion is compromised, the flow of undigested/unabsorbed nutrients into the hindgut increases(Reference Choct, Hughes and Wang45, Reference Jørgensen, Zhao and Eggum51) and the microbial ecology can change dramatically(Reference Hübener, Vahjen and Simon52). Bird et al. (Reference Bird, Vuaran and Brown61) compared pigs fed highly digestible starch (low amylose) and low digestible starch (mixture of non-heated and heated high amylose). Low starch digestibility had a dramatic effect on intestinal physico-chemical and microbial characteristics. The study also reported increased caecum weight and colon length in pigs fed low digestible starch and this was linked to a large flow of digesta into the hindgut that stimulated gut mass growth. These findings were replicated in a more recent pig study (Regmi et al. (Reference Regmi, Metzler-Zebeli and Gänzle62)) that showed that slowly digestible starch resulted in poor feed efficiency concomitant with an increased flow of DM, starch and protein in the hindgut. Dietary fibre and in particular soluble NSP have been shown to have dramatic effects on the flow of undigested DM into the hindgut. For example, Jørgensen et al. (Reference Jørgensen, Zhao and Eggum51) showed that increasing dietary fibre from 6 to 27 % resulted in a 5- to 6-fold increase in the flow of digesta through the terminal ileum of pigs. In broiler chickens, the addition of soluble NSP (40 g/kg) isolated from wheat to a sorghum-based diet reduced the ileal digestibility of starch and protein by more than 35 %(Reference Choct, Hughes and Wang45). The implications of increased concentration of undigested substrates in the GIT are such that the microbiota proliferates and competes with the host for the available nutrients, and increase the risk of microbial perturbation if the balance is tipped for pathogen growth(Reference Pluske, Pethick and Hopwood15). Furthermore, the host responds to increased undigested substrates by increasing the gut mass which carries a cost in terms of increased maintenance requirements which ultimately compromise efficiency of growth(Reference Ferrell63, Reference Agyekum, Slominski and Nyachoti64). It is inevitable that the use of any additive that influences the digestibility of the diet will change the selection pressures on the resident microbiota which in turn will moderate the efficiency with which the host utilises its feed.
Phytase
Phosphorous is the third most expensive nutrient in diets for non-ruminants; however, the majority (>65 %) of the phosphorous in feedstuffs of plant origin is bound in mixed salts of phytic acids and is unavailable to the animal without enzymic dephosphorylation(Reference Kiarie, Nyachoti, Vitti and Kebreab65). Phytase, the requisite enzyme to hydrolyse phytic acids, is insufficient in avian and mammalian pancreatic and intestinal secretions, present in some feedstuffs and ubiquitous in microbial systems(Reference Selle and Ravindran66, Reference Selle and Ravindran67). Consequently, to provide adequate phosphorous to non-ruminant farm animals it is necessary to include feedstuffs with high phosphorous availability such as inorganic supplements (for example, dicalcium phosphate) or animal-based feedstuffs (for example, meat and bone meal) in the diet. While this approach is plausible for adequate phosphorous nourishment, it creates three challenges: excessive excretion of phosphorous in the manure; expensive diets; and considerable demand on non-renewable global reserves for rock phosphate(Reference Kiarie, Nyachoti, Vitti and Kebreab65). Cognisant of the fact that the extent of the use of supplemental sources of phosphorous in the diets for non-ruminants depends on the digestible phosphorous content of the basal vegetable feedstuffs, research and commercial efforts have been directed at improving utilisation of phytic acid in these feedstuffs. To this end, the use of exogenous microbial phytase is almost ubiquitous in poultry and swine feeds and has received numerous reviews (for example, Adeola & Cowieson(Reference Adeola and Cowieson3), Slominski(Reference Slominski7) and Woyengo & Nyachoti(Reference Woyengo and Nyachoti6)).
Mixed salts of phytic acid can also exert anti-nutritional effects through the increased endogenous N losses and formation of complexes with proteins and other nutrients(Reference Selle and Ravindran66–Reference Yu, Cowieson and Gilbert72). For example, Onyango et al. (Reference Onyango, Asem and Adeola71) reported that phytic acid increased mucin and endogenous amino acid losses from the GIT of chickens. Several theories have been proposed as to the mechanism of how phytate binds to protein and reduce its digestibility. It is believed that at an acidic pH, a binary protein–phytate complex may form where phytate can bind to the α-NH2 groups and side chains of the basic amino acids arginine, histidine and lysine(Reference Selle, Cowieson and Cowieson68, Reference Cowieson, Acamovic and Bedford69). Indeed, in recent digestive tract simulation studies, Yu et al. (Reference Yu, Cowieson and Gilbert72) demonstrated that degradation of phytate to lower-level esters dramatically increased the solubility of soya and casein proteins.
Consequences of anti-nutritive effects of phytic acid are that ileal digestibility of dietary protein is reduced and endogenous secretions, in particular of mucin, are increased(Reference Cowieson, Acamovic and Bedford69). It is therefore plausible that phytic acid destruction through supplemental phytase would result in reduced endogenous losses and increased protein digestibility, thus limiting protein supply to the hindgut and exerting microbiota changes. There is, however, a dearth of information on the effects of phytase on the gut microbiome in single-stomached animals. Lumpkins et al. (Reference Lumpkins, Humphrey and Mathis73) showed that phytase reduced intestinal 5AC mucin mRNA abundance in broiler chickens. Clostridium thrives on mucin, and a reduction in mucin levels could correspond to a reduction in C. perfringens and the occurrence of NE(Reference Cooper and Songer74). Increased caecal acetate was reported in broiler chickens fed phytase, an indication of microbial activity modulation(Reference Smulikowska, Czerwinski and Mieczkowska75). In pigs, supplemental phytase was observed to increase Clostridium group in the ileum without changing total bacterial numbers(Reference Metzler-Zebeli, Vahjen and Baumgartel76) and Bifidobacterium and Clostridium numbers(Reference Wang and Lei77) in the ileum. In a more recent pig study, Rostagno et al. (78) observed that the addition of alkaline phosphatase to a phosphorous-deficient diet (80 % requirement) reduced the ileal digesta count of Enterococci, whereas the addition of alkaline phosphatase to a diet adequate in phosphorous reduced coliforms, aerobes and anaerobes. Interestingly, alkaline phosphatase had no effect on caecal or faecal microflora in both phosphorous-inadequate and -adequate diets. It is well documented that intestinal alkaline phosphatase is highly efficacious in dephosphorylating free bacterial lipopolysaccharides, a component of the outer membrane of Gram-negative bacteria(Reference Chen, Malo and Moss55, Reference Lallés56). The outer membrane in Gram-negative bacteria plays an important role in nutrient uptake and provides the organism with a remarkable permeability barrier, conferring resistance to a variety of detergents and antibiotics(Reference Doerrler79). In view of the foregoing, it can be speculated that dephosphorylation of the lipopolysaccharides within the bacterial outer membrane might compromise viability of the bacteria. However, in vitro experiments indicated that the exogenous intestinal alkaline phosphatase was unable to directly alter the growth, surface biology or invasiveness of live, intact bacteria(Reference Chen, Malo and Moss55, Reference Malo, Alam and Mostafa80). Few studies have investigated the effects of fed alkaline phosphatase on gut microbiota. For example, alkaline phosphatase promoted restoration of the normal gut microbiota following antibiotic exposure and inhibited Salmonella colonisation in mice; however, it also appeared to provide a favourable environment for E. coli growth(Reference Malo, Alam and Mostafa80). Malo et al. (Reference Malo, Alam and Mostafa80) opined that alkaline phosphatase may not exert its effects on the gut microbiota via direct mechanisms, but rather via mechanisms related to reduction of the mucosal microenvironment pH through luminal ATP dephosphorylation, altered inflammatory status following lipopolysaccharide detoxification or some other not yet known factors. Nonetheless, the foregoing pig studies seem to suggest that phytic acid-dephosphorylating enzymes make an impact on microbiota in the small intestine more than in the hindgut; however, the implications of such changes in terms of gut health are yet to be understood.
Carbohydrases and proteases
It has recently been reported that the beneficial effects of FE are inextricably linked to the amount of the undigested fat, protein and starch in the ileum(Reference Romero and Plumstead81). For example, Romero et al. (Reference Romero, Plumstead and Ravindran82) fed broiler chicken two types of diets, a simple maize–soya diet (maize-SBM) and a complex diet in which maize-SBM was fortified with dried distillers grains with solubles and rapeseed meal in line with the current trends in the industry to seek alternative ingredients to curb feed costs. The results (Fig. 2) showed that a blend of xylanase, amylase and protease improved ileal digestibility of starch, fat and protein and thus significantly improved the bird's ability to extract energy in two distinctly different diets in terms of substrate complexity. Such accelerated intestinal digestion and removal of what would otherwise be apparently undigested without FE must clearly limit the nutrients available for the microbes.

Fig. 2 Ileal digestibility (%) of starch (A), fat (B), protein (C) and their contribution (kJ/kg diet) to dietary energy (D) in maize–soya (maize-SBM) or maize–soya diets with 10 % dried distillers grains and 5 % rapeseed meal (mixed) fed to broiler chickens as influenced by enzyme combinations containing xylanase and amylase (XA), or xylanase, amylase and protease (XAP). (D) ![]() , Protein;
, Protein; ![]() , fat;
, fat; ![]() starch. Xylanase was from Trichoderma reesei at 2000 units/kg feed; amylase was from Bacillus lichiniformis at 200 units/kg feed; protease was from B. subtilis at 4000 units/kg feed. a,b,cMean values with unlike superscript letters within a subgrouping were significantly different (P< 0·05). Adapted from Romero et al. (Reference Romero, Plumstead and Ravindran82) (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr).
starch. Xylanase was from Trichoderma reesei at 2000 units/kg feed; amylase was from Bacillus lichiniformis at 200 units/kg feed; protease was from B. subtilis at 4000 units/kg feed. a,b,cMean values with unlike superscript letters within a subgrouping were significantly different (P< 0·05). Adapted from Romero et al. (Reference Romero, Plumstead and Ravindran82) (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr).
Indeed, Torok et al. (Reference Torok, Ophel-Keller and Loo53) used the terminal restriction fragment length polymorphism method to examine changes in gut microbial communities in response to the addition of an NSP-degrading enzyme product containing β-glucanase, xylanase and protease activities in a barley-based diet. The enzyme product improved growth performance and energy utilisation compared with the control. Further correlation analysis of the dietary apparent metabolisable energy and microbial community composition within the ileum and caeca revealed distinct clusters associated with control and enzyme-supplemented birds. This is one of few studies that directly linked differences in the composition of the gut microbial community with improved performance, implying that the presence of specific beneficial and/or absence of specific detrimental bacterial species may have contributed to the improved performance in birds receiving FE. Furthermore, this study is a clear indication that molecular techniques coupled with statistical methods are capable of identifying desirable and undesirable clusters of organisms as far as good performance is concerned. Previous reports showed that supplemental carbohydrases reduced caecal counts of Campylobacter jejuni in broilers(Reference Fernandez, Sharma and Hinton50) and Brachyspira intermedia in the laying hen(Reference Hampson, Phillips and Pluske83). Clearly, FE might have dramatically altered the caecum ecology to the extent that for these specific bacteria growth was not favourable.
Short-chain oligosaccharides with potential prebiotic effects
Usage of NSP-degrading enzymes (NSPases; xylanase, β-glucanase, β-mannanase, α-galactosidase and pectinase) is common in poultry and swine feeds. As reviewed by Simon(Reference Simon84), NSPases targeting NSP may have several modes of action: partial hydrolysis of NSP, decrease in digesta viscosity, and rupturing of NSP-containing cell walls, thereby making the encapsulated nutrients available for digestion. Partial hydrolysis of soluble and insoluble NSP and rupturing of NSP-containing cell walls have been associated with reduced recovery of NSP when feedstuffs were incubated with NSPases(Reference Castanon, Flores and Pattersson85, 86). Furthermore, studies investigating the efficacy of NSPases reported not only improvement of nutrient digestibilities but also digestibility of NSP(Reference Adeola and Cowieson3, Reference Slominski7). Although there are some studies in which no effect of added NSPases on nutrients digestibility was recorded, for example, as reviewed by Adeola & Cowieson(Reference Adeola and Cowieson3), if the added NSPases in the diet do achieve the intended purpose they do so by partially hydrolysing the substrate, i.e. dietary NSP. It is possible that part of the variation in the response of NSPases on NSP utilisation is related to interactions with the gut microbiota, which have a role in the utilisation of undigested substrates in the intestinal lumen.
In commercial human food production, short-chain oligosaccharides are extracted from natural sources as is the case for the galacto-oligosaccharides from soyabeans, but they can also be obtained biochemically(Reference Voragen87). Indeed, in the food industry, enzymic hydrolysis processes are applied for production of a whole array of oligosaccharides from plant cell wall polysaccharides(Reference Voragen87, Reference Vázquez, Alonso and Domínguez88). As outlined by Voragen(Reference Voragen87), the concept for manufacturing oligosaccharides from suitable polysaccharides is simple: starting from a polysaccharides-rich feedstock followed by controlled hydrolysis of some of the heterocyclic ester bonds of the main chain backbone by an exogenous enzyme to give compounds of a lower degree of polymerisation (DP). For example, the enzymic hydrolysis process is widely applied in the commercial production of fructo-oligosaccharides from inulin(Reference Chesson, Stewart, Piva, Bach Knudsen and Lindberg89). Manufactured sources of oligosaccharides may allow the dose to be more precisely defined and controlled, but only a very limited range of oligosaccharide structures is presently available. Most are linear, with short chain length and containing only one or two different sugar units, and are specially manufactured for the ever-increasing human market.
Plant and plant co-products used by the feed industry contain almost an unlimited range of polysaccharides (Table 1). The use of specific NSPases, singly or in combination, against a range of polysaccharides can generate very large numbers of oligomer mixtures(Reference Chesson, Stewart, Piva, Bach Knudsen and Lindberg89). However, the NSP listed in Table 1 represent general classes in which there is commonality of overall structure but considerable variation in fine structure. For example, while the underlying structure of most arabinoxylans is similar, i.e. a β-1,4-linked backbone of d-xylose residues, in practice the variety is enormous due to differences in backbone size and in type and degree of substitutions from the backbone, all of which depend on the source of arabinoxylan(Reference Sunna and Antranikian90). For example, xylan hydrolysis products from maize are different from those produced from wheat in terms of the size, degree of substitution and in quantity(Reference Hespell, O'Bryan and Moniruzzaman91, Reference Li, Azadi and Collins92). Even when the source of polysaccharide is constant (i.e. same grain), fine structure can vary with age, environmental conditions of growing and between varieties. For example, Austin et al. (Reference Austin, Wiseman and Chesson93) showed that partial degradation of arabinoxylan from twelve UK-grown wheat samples with an endoxylanase (single cloned) resulted in mixtures of up to twelve oligosaccharides, with each differing in the presence and relative proportions of these oligomers. Such an observation suggested that even when the enzyme has only one activity, it is likely that polysaccharides will uniquely release a mixture of oligomers. Importantly, such divergence in products of enzyme activity will probably influence the in vivo response observed.
Table 1 Carbohydrate contents (g/kg DM) and major NSP in common feedstuffs*
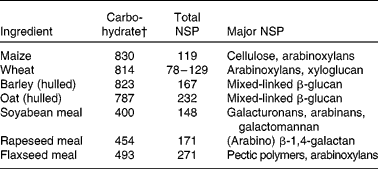
* Adapted from Bach Knudsen(Reference Bach Knudsen9, Reference Bach Knudsen103) and Meng(86).
† Includes lignin.
Regardless of whether a crude or purified enzyme product is used to hydrolyse NSP in typical diets for non-ruminants, it follows that short-chain oligosaccharides must be generated on every occasion when NSPases are used as feed additives. In this context, Apajalahti & Bedford(Reference Apajalahti and Bedford94) showed that the addition of xylanase in wheat-based broiler diets resulted in a 5-fold increase in concentrations of short-chain xylo-oligomers ( < 10 DP) in the caecum. There are apparently few studies where the in situ generation of oligosaccharides has been monitored in pigs and chickens fed diets containing NSPases. However, since oligosaccharides will be among the hydrolysis products released when NSPases degrade NSP, a question has been raised as to whether the presence of such products would influence GIT ecology(Reference Pluske, Pethick and Hopwood15, Reference Chesson, Stewart, Piva, Bach Knudsen and Lindberg89, 95). This is because the resulting NSP hydrolysis products may modulate intestinal microflora(Reference Pluske, Pethick and Hopwood15, Reference Chesson, Stewart, Piva, Bach Knudsen and Lindberg89). Numerous reports in pigs indicated that supplemental NSPases increased intestinal SCFA and this was associated with increased abundance and activity of specific bacteria communities such lactobacilli(Reference Diebold, Mosenthin and Piepho96–Reference Kiarie, Nyachoti and Slominski98). In chickens, abundance of Enterobactreacea was reduced in caeca when birds were offered cereal-based diets with supplemental NSPases(Reference Rosin, Blank and Slominski99, Reference Jòzefiak, Rutkowski and Kaczmarek100). Furthermore, Choct et al. (Reference Choct, Hughes and Wang45, Reference Choct, Hughes and Bedford101) linked increased concentration of SCFA in the caecum of chickens fed diets with supplemental xylanase to increased flow of xylo-oligomers into the caecum. However, as the in situ production of short-chain oligosaccharides was not monitored in the aforementioned studies, it is thus rather difficult to associate observed microbial activity with the presence or absence of NSP hydrolysis products.
Short-chain xylo-oligosaccharides (arabinoxylooligosaccharides; AXOS) derived from the in vitro hydrolysis of wheat bran with an endoxylanase and fed to broiler chickens resulted in increased bifidobacteria populations in the caecum and improvements in feed conversion ratio (FCR) on both wheat- and maize-based diets(Reference Courtin, Broekaert and Swennen102). Interestingly, in the same study some birds were fed diets supplemented with xylanase instead of AXOS and the scale of the improvement in FCR achieved was equal for both strategies. This suggests that the production of fermentable oligomers may well be a large part of the total response to FE supplementation, although it was noted that the xylanase did not influence the intestinal flora to the same extent as that of the oligosaccharides, suggesting the xylanase, at the levels used, may not have released similar quantities of AXOS in situ. As the majority of arabinoxylans in feedstuffs are insoluble(Reference Bach Knudsen103), perhaps the xylanase used was not as effective in solubilising and cleaving insoluble NSP to effective oligomer sizes under limiting gut conditions (for example, digesta transit time). The effects on FCR were of similar magnitude in both the maize- and wheat-based diets, suggesting that caecal fermentation may be of significance. Xylo-oligosaccharides are not digestible or are very poorly digestible up to the ileum–caecum junction, but are fermented in part by microbiota in the caecum with concomitant production of SCFA and thus provision of extra energy to the birds. This might partly explain the reason why FCR improved with feeding AXOS in both maize- and wheat-based diets. It is also possible that AXOS improved the uptake of minerals in the caecum, as has been observed for inulin via lower pH caused by enhanced fermentation(Reference Van Loo104). The production of butyrate, a fuel for the colonocytes, might have also improved the absorptive capacity of the intestinal mucosa(Reference Guilloteau, Martin and Eeckhaut105). Furthermore, these data indicated that AXOS modulated gut health by increasing bifidobacteria counts in line with observations in other animal models(Reference Damen, Verspreet and Pollet106); a benefit of bifidogenic effects is overall better gut health and thus better FCR. This observation perhaps suggested decreased energetic costs associated with constitutive low-level inflammation caused by enteropathogens or caused by reduced passage of pro-inflammatory components of (commensal) bacterial origin such as lipopolysaccharides(Reference Anderson, McCracken and Aminov107). However, it remains to be proven that AXOS are released by xylanases in the GIT at levels sufficient to exert microbiota-modulating effects.
A potential limitation for in situ generation of AXOS via supplemental NSPases such as xylanase is the fact that xylanases are inhibited by the presence of AXOS (negative feedback), and the shorter the chain length the greater the inhibition, so in many cases the depolymerisation of xylan is self-limiting(Reference Lo and Pickersgill108), with the result that in situ production of AXOS in the gut might be limited. However, a recent study in rats indicated that the prebiotic potential and fermentation characteristics of cereal AXOS depend strongly on their structural properties(Reference Damen, Verspreet and Pollet106). The study reported that feeding a mixture of arabinoxylans of different DP (5–284) resulted in a larger increment in bifidobacteria in the caecum without increasing total bacteria; however, SCFA levels were greater for AXOS with 284 DP than for AXOS of 5 DP. Furthermore, evaluation of structurally different AXOS showed that smaller AXOS resulted in higher intestinal butyrate concentrations and a pronounced bifidogenic effect, whereas larger compounds mainly led to lower branched SCFA concentrations, an indication of lower proteolytic fermentation (i.e. better indices of gut health)(Reference Van Craeyveld, Swennen and Dornez109). These insights into the structure–activity relationship of AXOS suggest tremendous opportunity for developing and evolving FE products capable of in situ production of AXOS with optimised prebiotic and fermentation properties. Furthermore, perhaps future research could explore co-supplementation of NSPases/AXOS with probiotics to synchronise production of oligomers and utilisation.
Influence of feed enzymes on the gastrointestinal health–pathogen challenge: the case for a disease challenge model
Choct et al. (Reference Choct, Sinlae and Al-Jassim110) reported that broiler chickens fed a wheat-based diet with xylanase had a negligible number of C. perfringens compared with control birds. Nian et al. (Reference Nian, Guo and Ru111) showed that the addition of xylanase to a wheat-based diet improved performance and was accompanied by a reduction in the number of coliforms and Salmonella in the ileum. In piglets, there was a significant reduction in the frequency and severity of diarrhoea in piglets fed diets supplemented with fibre-degrading enzymes(Reference Inborr and Ogle112). However, the cause of diarrhoea was not determined, which presents problems in interpreting these data, as the diarrhoea seen after weaning can be osmotic(Reference Pluske, Pethick and Hopwood15) in nature rather than being of bacterial origin which might be influenced by FE(95). The presence or absence of a pathogenic organism may not necessarily predict that disease will occur unless pathogen numbers proliferate to such an extent to overwhelm the normal microbial population in the GIT. However, the study of a specific bacterial disease, particularly if it causes economic loss, offers a means of assessing the usefulness of nutritional interventions or strategies on the survival of that particular pathogen in the GIT, and its subsequent effects on production, morbidity and mortality(Reference Pluske, Pethick and Hopwood15).
There are a number of well-known enteric bacterial diseases that occur throughout the world, and each is relatively unique in that it generally occurs at different phases of pig and chicken growth and/or in different regions of the GIT(Reference Pluske, Pethick and Hopwood15, Reference Timbermont, Haesebrouck and Ducatelle32). Enteric diseases are an important concern to the poultry and pork industries because of production losses, increased mortality, reduced welfare of animals and increased risk of contamination of poultry and pork products for human consumption. Numerous reports have used experimental enteric pathogen challenge models to examine whether a particular feed additive or dietary strategy is effective in controlling pathogens under study(Reference Jia, Slominski and Bruce31, 95). A major advantage of using such an in vivo model is that the impact of the particular additive can be assessed in a context of an infectious pathogenic agent being part of the GIT ecosystem. The following sections deal with four enteric diseases that afflict chickens and pigs worldwide and report examples of investigations exploring how FE can modulate the expression of the pathogen and accompanying disease that can occur.
Post-weaning colibacillosis in piglets
Post-weaning colibacillosis is a disease largely afflicting the small intestine and is caused by certain enterotoxigenic strains of E. coli (ETEC). Despite a rapid digesta flow through the small intestine(Reference Turlington, Allee and Nelssen113), the pathogenic E. coli possess fimbriae, or pili, that attach to the enterocytes lining the small-intestinal villi or to the mucus covering the villi(Reference Fairbrother, Nadeau and Gyles23). This attachment physically prevents the bacteria from being flushed through to the large intestine. E. coli fimbriae attach to glycoprotein receptors expressed in the brush border of cells lining the intestinal villi. The most common E. coli associated with causing post-weaning colibacillosis is K88, renamed as F4(Reference Fairbrother, Nadeau and Gyles23). After colonising the small intestine, ETEC provoke hypersecretory diarrhoea through the release of specific enterotoxins (heat-labile toxins) that cause secretion of ions and water into the lumen. Post-weaning colibacillosis is a major cause of mortality and morbidity worldwide(Reference Fairbrother, Nadeau and Gyles23). Colonisation of the small intestine and diarrhoea usually lasts between 4 and 14 d, with the strains being spread between animals primarily by the faecal–oral route, and also by aerosols(Reference Fairbrother, Nadeau and Gyles23). However, it is important to note that despite ETEC being identified as the primary infectious agent in this disease, there is abundant evidence to suggest that a plethora of other factors are necessary for post-weaning colibacillosis to occur(Reference Fairbrother, Nadeau and Gyles23). Given the economic importance of post-weaning colibacillosis to pig production, diets fed to newly weaned pigs generally contain antimicrobial agents (antibiotics or ZnO) to control post-weaning diarrhoea and reduce the negative impacts of this disease. Restriction on the use of antimicrobial agents in some parts of the world, however, has forced the pig industry to examine other ways of controlling this disease, with ‘nutrition’ being a prime and obvious area of investigation.
Thus, Kiarie et al. (Reference Kiarie, Slominski and Krause114, Reference Kiarie, Slominski and Nyachoti115) showed that when certain feedstuffs were incubated with a mixture of enzymes (xylanase, cellulases and mannanase) a wide range of sugars was released (Table 2). It is interesting to note that mannose was released during the hydrolysis of soyabean meal and wheat middlings; mannose is a well-described immune stimulant(Reference Jensen, Patterson and Yoon116). This indicated that when these ingredients were subjected to enzyme hydrolysis, potential exists for active prebiotic release. The potential of such enzyme hydrolysis products in influencing the secretory diarrhoea caused by ETEC K88 was assessed in an in situ model of secretory diarrhoea(Reference Nabuurs, Hoogendoorn and van Zijderveld117). In this context, the enzyme hydrolysis products were infused into small-intestinal segments prepared in live anaesthetised pigs and experimentally infected with ETEC and fluid passage through the segments measured. The segments that were infused with soyabean, wheat or flaxseed hydrolysis products had greater fluid absorption than control segments that were infused with an isotonic saline solution. What this means is that the hydrolysis products allowed the intestinal segments to retain more fluid and mitigate the negative effects of the ETEC infection. The fact that soyabean meal and wheat middlings had the greatest effects suggests that mannose mediated these effects, thus corroborating previous evidence that products derived from partial enzymic hydrolysis of wheat arabinoxylans(Reference Alam, Sarker and Molla118) and fermented soyabeans(Reference Kiers, Meijer and Nout119–Reference Kiers, Nout and Rombouts121) enhanced fluid and electrolyte balance in models of intestinal secretory diarrhoea.
Table 2 Monomeric sugar composition (mg/g) of enzyme hydrolysis products from common feedstuffs*
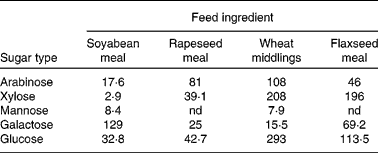
nd, Not detected.
* Adapted from Kiarie et al. (Reference Kiarie, Slominski and Krause114, Reference Kiarie, Slominski and Nyachoti115).
The enzyme hydrolysis products were further tested in piglets fed either a control diet (devoid of ingredients used to generate the hydrolysis products) or a treatment diet with composite hydrolysis products from wheat middlings, soyabean meal, rapeseed meal and flaxseed at 5 g/kg(Reference Kiarie, Slominski and Krause122, Reference Kiarie, Slominski and Krause123). Piglets received these diets for 9 d during which basic performance metrics were measured. After this initial period, all pigs were orally challenged with ETEC and monitored for another 48 h. During the pre-challenge period piglets receiving diets with hydrolysis products ate more (299 v. 269 g/d) and grew faster (252 v. 207 g/d) than control piglets. Furthermore, during the challenge period, piglets fed hydrolysis products consumed more feed, suggesting the ability of enzyme hydrolysis products to attenuate ETEC enteritis, the hallmark of anorexia during infections (Fig. 3(A)). This peculiarity is explained by the lower incidence of diarrhoea (Fig. 3(B)) observed for the pigs fed the hydrolysis products. Interestingly, the piglets fed the enzyme hydrolysis products had lower stomach pH (2·62 v. 3·86) than the control piglets. Maintaining low gastric pH is important in the control of enteric pathogens such as ETEC which are transmitted within the herd via oral–faecal cycling(Reference Fairbrother, Nadeau and Gyles23). The enzyme hydrolysis products mediated this effect by eliciting overall high fermentation as measured by increased organic acid production and in particular lactic acid concentration. Foregoing research indicates that enzyme hydrolysis products of common piglet feedstuffs were beneficial in minimising the negative effects of ETEC infection, affording the piglet a healthier gut as indicated by better appetite in the presence of active disease. It is relevant that viscous fibres have been linked with the exacerbation of colibacillosis in some studies(Reference Hopwood, Pethick and Pluske35, Reference Montagne, Cavaney and Hampson36). It appears that there is tremendous potential in developing FE for degrading NSP to generate short-chain oligosaccharides capable of controlling enteric infections such as ETEC-secretory diarrhoea.
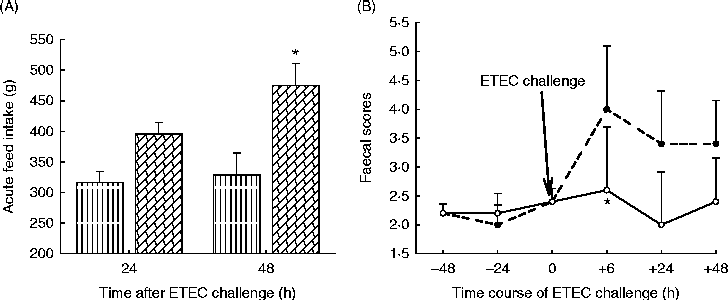
Fig. 3 Acute feed intake of piglets fed enzyme hydrolysis products (![]() ) or a control diet (
) or a control diet (![]() ) and challenged with enterotoxigenic Escherichia coli (ETEC) (A) and incidence and severity of diarrhoea (faecal scores) in piglets fed enzyme hydrolysis products (
) and challenged with enterotoxigenic Escherichia coli (ETEC) (A) and incidence and severity of diarrhoea (faecal scores) in piglets fed enzyme hydrolysis products (![]() ) or a control diet (
) or a control diet (![]() ) upon challenge with ETEC (B). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control (P< 0·05). Adapted from Kiarie et al. (Reference Kiarie, Slominski and Krause122, Reference Kiarie, Slominski and Krause123).
) upon challenge with ETEC (B). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control (P< 0·05). Adapted from Kiarie et al. (Reference Kiarie, Slominski and Krause122, Reference Kiarie, Slominski and Krause123).
Swine dysentery
SD is a mucohaemorrhagic colitis occurring mainly in growing–finishing pigs. It affects the caecum, colon and rectum and is caused by the anaerobic spirochaete Brachyspira (Serpulina) hyodysenteriae (Reference Harris, Hampson, Glock, Straw, D'Allaire, Mengeling and Taylor124). Clinical manifestations vary considerably, but infected pigs typically develop diarrhoea, which initially is grey to black and sometimes watery, and progresses to consist of mucus plugs, fibrin, epithelial cell casts and flecks of fresh blood(Reference Harris, Hampson, Glock, Straw, D'Allaire, Mengeling and Taylor124). In many countries, SD is one of the most economically important endemic bacterial diseases of swine during the grow-out period(Reference Pluske, Pethick and Hopwood15). It is relevant that recent reports appear to indicate the emergence of SD in North America and anecdotal evidence appears to link this emergence to high usage of high-fibre co-products such as dried distillers grains with solubles(Reference Schwartz125).
Many factors have been implicated in the aetiology of SD(Reference Harris, Hampson, Glock, Straw, D'Allaire, Mengeling and Taylor124) and there is compelling evidence that diet may modulate the expression of the disease. Numerous hypotheses have been advanced in an attempt to explain dietary effects on SD. These include dietary influence on the survival of spirochetes(Reference Siba, Pethick and Hampson126), motility of the spirochete in the mucosal lining and chemotaxic-regulated motility(Reference Kennedy, Rosnick and Ulrich127), ability of B. hyodysenteriae to express haemolysins and lipopolysaccharides that cause inflammation of the epithelium(Reference Zhang, Duhamel and Mysore128). Pluske et al. (Reference Pluske, Durmic and Pethick40) showed that feeding a highly digestible diet based on cooked rice and high-quality proteins was more protective than a diet based on wheat, barley and sweet lupins. These authors surmised that a highly digestible diet reduced the degree of hindgut fermentation and reduced both the proliferation of B. hyodysenteriae and clinical expression of the disease as opposed to wheat, barley and sweet lupins that promoted hindgut fermentation (as evidenced by lower pH, increased SCFA levels, heavier organ weights). As alluded to, FE reduce the undigested components flowing into the hindgut and might also generate short-chain oligosaccharides that can modulate overall fermentation in the gut. It is therefore logical to expect that FE might partly modulate the expression of SD by reducing the load of fermentable substrate entering the large intestine.
This hypothesis was tested by Durmic et al. (Reference Durmic, Pethick, Mullan and Cranwell38) who fed extruded or non-extruded wheat without or with xylanase supplementation. Pigs were challenged with a virulent strain of B. hyodysenteriae and subsequently monitored for expression of the disease. As expected, both extrusion of wheat and addition of xylanase increased pre-caecal starch digestion, as indicated by reduced starch levels in the large intestine. Furthermore, xylanase in non-extruded wheat improved growth performance, extending compelling evidence of the efficacy of xylanase in wheat-based diets. Xylanase increased microbiota activity and thus fermentation in the proximal areas of the hindgut, as corroborated by an increase in bacterial ATP concentrations. However, a peculiar observation in the study is the significant main effect of xylanase on digesta pH such that in the distal part of the colon, pigs fed xylanase had a higher pH than pigs not fed xylanase. These data suggested that xylanase accelerated the utilisation of carbohydrates in the proximal sections of the gut such that by the time the digesta reached the distal colon, N dominated as the fermentation substrate. An area that is not well researched is the impact of dietary protein and type and expression of SD(Reference Pluske, Pethick and Hopwood15). Given the postulated effects of excess protein entering the caecum and colon on bacterial proliferation and production of bacterial metabolites(Reference Heo, Opapeju and Pluske27), it is feasible that this component of the diet might also influence the aetiology of SD. Perhaps an overall strategy should employ combination of NSPases and protease for synchronised carbohydrate and N disappearance in the upper gut. Furthermore, when assessing efficacy of FE in controlling SD, it is important to recognise that bacterial species, such as Fusobacterium spp., Clostridium spp. and Bacteroides spp., need to be present in sufficient numbers for SD to occur(Reference Harris, Hampson, Glock, Straw, D'Allaire, Mengeling and Taylor124). It is therefore imperative for future studies to incorporate community profiling using 16S rDNA sequence analysis to monitor potential inadvertent microbiome changes likely to implicate FE in B. hyodysenteriae challenge studies.
Salmonellosis
Subclinical Salmonella enterica infections in pig herds and chicken flocks are recognised as important sources of human salmonellosis and hence a potential threat to human health through food-borne disease outbreaks(Reference Gast129). Post-harvest measures are of importance to reduce the contamination of carcasses, but measures at the primary production site are still necessary to prevent transmission of Salmonella strains to the slaughterhouse. Although hygienic practices are important to avoid the introduction of Salmonella on the farm or reduce infection pressure when Salmonella is present, these practices do not sufficiently reduce Salmonella contamination on the farm. Feed additives constitute an important group of pre-harvest measures that can help in controlling Salmonella on the farm. Feed additives used for the control of Salmonella can be of different types, including antibiotics, prebiotics, probiotics and synbiotics(Reference Van Immerseel, Eeckhaut and Teirlynck130). An important role that feed additives can play is in reducing the infection pressure and thus limiting the risk of contamination of poultry and pork products. Among the non-antibiotic solutions, prebiotics have received much interest in controlling Salmonella infections in poultry(Reference Van Immerseel, Eeckhaut and Teirlynck130).
Eeckhaut et al. (Reference Eeckhaut, Van Immerseel and DeWulf131) evaluated the influence of two AXOS differing in DP on shedding and colonisation of S. enteritidis in broilers upon challenge. The AXOS products were included in the control diet at 0·2 % for 3-DP-AXOS and 0·2 % and 0·4 % for 9-DP-AXOS. The AXOS attenuated Salmonella colonisation as shown by lower excreta shedding and concentration in the caecum tissues and systemic translocation to the spleen. This protection also seemed to be dose dependent, as the level of protection for all examined tissues was greater in the chicks receiving 0·4 % 9-DP-AXOS compared with those receiving 0·2 % of 9-DP-AXOS. Furthermore, the average chain length appeared to play a role, because, in general, less Salmonella colonisation and translocation were observed with 0·2 % 9-DP-AXOS than with the same dose of 3-DP-AXOS. These results were reproduced in birds fed xylanase and challenged with Salmonella (Reference Vandeplas, Dauphin and Thiry132). These authors showed that the FCR of challenged birds fed xylanase was better than challenged control birds and commensurate with that of the non-challenged group. Interestingly, the responses were even greater when xylanase was fed in combination with a lactobacilli-based probiotic.
In a more recent Salmonella challenge study, Amerah et al. (Reference Amerah, Mathis and Hofacre133) showed that xylanase and essential oil supplementation reduced Salmonella-positive caecal samples in broiler chickens by 61 and 7·7 %, respectively, compared with the control. Although combination xylanase and essential oil did not show synergistic benefits on influencing Salmonella colonisation, potential complementary effects of FE and additives such as essential oils and probiotics hold tremendous opportunity in controlling common enteric pathogens through reduced overall bacterial populations in the gut and the attendant benefit of decreased nutrient competition with the host and inflammatory pressure. Recent industry analysis revealed that Salmonella vaccination programmes have not consistently been able to prevent infection entirely (especially against high pathogen doses) or to effectively cross-protect against different serotypes(Reference Gast129). It is therefore relevant that FE have the potential of complementing Salmonella vaccination programmes as a strategy for controlling pre-harvest salmonellosis in the poultry industry.
Necrotic enteritis
NE is considered one of the most threatening diseases in the broiler industry worldwide(Reference Timbermont, Haesebrouck and Ducatelle32). The total global economic loss as a consequence of NE outbreaks in broiler farms is estimated to be over US $2 billion annually(Reference Ducatelle and Van Immerseel134). The causative agent of NE is C. perfringens, a Gram-positive spore-forming anaerobe. In broilers, NE presents itself as a sudden increase in mortality occurring at any time when the birds are between 2 and 6 weeks old. Mortality may reach up to 1 % per d and if left untreated may continue for 1–2 weeks(Reference Ducatelle and Van Immerseel134). In the last few years, a subclinical form of the disease has become more prevalent. It is characterised by poor digestion, reduced weight gain and increased FCR, without obvious increase in mortality(Reference Timbermont, Haesebrouck and Ducatelle32, Reference Ducatelle and Van Immerseel134). The hallmark of this disease is the presence of typical necrotic lesions particularly in the mid-region of the intestinal tract. Just like other enteric pathogens, many factors influence C. perfringens colonisation in the gut and subsequent expression of subclinical and clinical symptoms(Reference Timbermont, Haesebrouck and Ducatelle32, Reference Williams135).
Gut ecology that favours growth of C. perfringens has been recognised as one of the key risk factors for the development of NE. In particular, the nature of the diet has been shown to be an important factor that influences the incidence of NE(Reference Timbermont, Haesebrouck and Ducatelle32); for example, diets with high levels of indigestible water-soluble NSP(Reference Kaldhusdal and Skjerve30, Reference Jia, Slominski and Bruce31). It is therefore relevant that the use of any additive such as FE that influences the digestibility and degradation of NSP may be useful in mitigating the proliferation of C. perfringens. This hypothesis was tested by Jia et al. (Reference Jia, Slominski and Bruce31), who studied effects of diet type (maize v. wheat), multi-carbohydrase enzyme supplementation (without or with) and C. perfringens challenge (none and challenged) in broiler chickens. Growth performance, intestinal population of C. perfringens and gut lesions were among the responses evaluated. Birds fed maize-based diets had better FCR than those fed wheat-based diets. Pathogen challenge greatly impaired growth performance and increased intestinal C. perfringens counts and lesions. Enzyme supplementation minimised growth suppression associated with the pathogen challenge, with the most pronounced effect observed in birds fed the wheat-based diet. Instructively, wheat-based diets were observed to have induced high jejunal digesta viscosity that was reduced by enzyme addition. Similarly, an earlier study by Riddell & Kong(Reference Riddell and Kong29) observed mortality due to NE to be higher among broiler chickens fed rations based on wheat, rye, barley and oats than among chickens fed maize-based rations upon challenge with C. perfringens. However, these authors could not find any beneficial effect of xylanase supplementation on the susceptibility to NE in broilers fed wheat-based diets.
An important factor worth considering in reproducing NE in an experimental challenge with C. perfringens is that there is compelling evidence that C. perfringens and Eimeria spp. act synergistically in inducing NE lesions. In this context, co-infection with C. perfringens and Eimeria oocysts or an overdose of commercial coccidiosis vaccines containing attenuated Eimeria strains has been shown to result in more birds with lesions or in higher mortality rates compared with birds receiving only Eimeria or only C. perfringens (Reference Timbermont, Haesebrouck and Ducatelle32, Reference Williams135). The peculiarity is that Eimeria parasites kill intestinal epithelial cells as a consequence of the intracellular stages of their lifecycle(Reference Williams135). As a consequence, plasma proteins leak into the gut lumen through the resulting gaps in the epithelial lining of the intestinal lumen, and these serve as a growth substrate for C. perfringens strains(Reference Ducatelle and Van Immerseel134, Reference Williams135). It is also likely that the resulting malabsorption of nutrients due to the damage in the intestinal lining increases substrates for pathogen growth. Moreover, coccidial infection induces a T-cell-mediated inflammatory response that enhances intestinal mucogenesis(Reference Williams135). This enhanced mucin production provides a growth advantage to C. perfringens due to its ability to use mucus as a substrate(Reference Cooper and Songer74, Reference Collier, Hofacre and Payne136). In view of the foregoing and for the purpose of clarity in data interpretation, it is imperative to assess the effect of FE in modulating C. perfringens challenge within a context of a commercial coccidiosis vaccine. For example, Jackson et al. (Reference Jackson, Anderson and Hsiao137) used a NE disease challenge model involving oral inoculation of Eimeria spp. and C. perfringens and reported that β-mannanase was as effective as antibiotics in mitigating the disease effects as measured by growth performance and the incidence of coccidial lesion scores compared with the control. This study suggests that FE can play a role in controlling NE in circumstances where the use of antibiotics is not desired.
A core microbiome at a functional level
Reduction of undigested substrates in the small intestine and provision of fermentable NSP hydrolysis products are perhaps the probable means by which FE can affect gut microbiota. This is more so in instances where anti-nutrients such as phytic acids and viscous ingredients attenuate digestion such that significant quantities of starch and/or protein enter the large intestine, stimulating the activity of putrefactive bacteria and pre-disposing the animal to intestinal disorders. Clearly, under such circumstances the use of FE will improve small intestine digestion and as a result limit substrate availability in the large intestine, effectively mitigating any potential GIT microbial dysfunction(Reference Jia, Slominski and Bruce31, Reference Durmic, Pethick, Mullan and Cranwell38, Reference Inborr and Ogle112). However, it is still unclear whether, on feeding FE, the shifts in microbial profiles in the hindgut have a greater impact on animal health and nutrition than shifts in ileal profiles. Indeed, in some cases there may be significant changes in hindgut or ileal microbial profiles with little or no associated response in performance, indicating that the two are not always linked(Reference Parker, Oviedo-Rondon and Clack138). Nevertheless, it is clear that the response of the microflora to FE probably depends on the initial GIT microbial status, which in turn depends on the form and digestibility of the diet and the extent of the microfloral challenge it evokes. Many of the factors governing the extent of FE responses are most probably similar to those influencing responses to in-feed antimicrobials. For example, the response to antimicrobials in the diet depends to a large degree on the conditions under which the animals are raised(Reference Gaskins, Lewis and Southern13, Reference Niewold58). It is likely that any positive responses to FE will be dictated not only by the status of the microflora in the GIT, but also by environmental, management and dietary factors such as cereal type and quality, and processing(Reference Adeola and Cowieson3, Reference Bedford and Cowieson139).
By fair estimation most pigs and chickens are fed mostly non-viscous maize-based diets; furthermore, quite a great deal of advances have been made in housing equipment and bio-security. Under such production systems it might be difficult for a nutritionist to economically justify an extra dose of FE to cater for gut health. However, a key metric for profitability in swine and poultry production is growth performance, which in turn is dependent on variability in animal health and efficient utilisation of feed. At times this variability is driven by genetics and it is therefore likely that responses to FE may also depend upon the genotype of the animal. For example, Garcia et al. (Reference Garcia, Gomez and Mignon-Grasteau140) observed that xylanase use in birds selected over five generations for divergent digestion efficiency attenuated bile acid deconjugation (a measure of deleterious microbiota in the small intestines) in the less but not in the more efficient birds. But even so, when similar genotypes are fed the same diets variability in performance is expected and is indeed a major challenge for production systems employing all-in and all-out flock or herd flow management.
FE have a role in increasing uniformity of the flock or herd and thus improving profitability. However, the application of FE to manage poor-performing animals in a herd or flock would perhaps be more precise if such variability impinges on gut microbiota. Indeed, there is growing evidence linking dietary nutrient utilisation, growth performance and GIT microbiota composition(Reference Torok, Hughes and Mikkelsen17, Reference Torok, Ophel-Keller and Loo53, Reference Aphalajalati, Geier and Hughes141). Classical work by Turnbugh et al. (Reference Turnbaugh, Hamady and Yatsunenko142) demonstrated that a diversity of GIT microbiota assemblages can yield a core microbiome at a functional level, and that deviations from this core are associated with different physiological states (obese v. lean). This study corroborated earlier findings in mice by Turnbaugh et al. (Reference Turnbaugh, Ley and Mahowald143) and collectively demonstrates that the microbiomes in obese mice and human individuals harbour a larger proportion of genes for digesting fat, protein and carbohydrates, which might make them better at extracting and storing energy from food. As the application of molecular microbiology techniques increases in animal nutrition research, a potential strategy can be the establishment of robust and repeatable associations between enrichment of particular strains or clusters of bacteria and animal performance. In this context, recent studies by Torok et al. (Reference Torok, Hughes and Mikkelsen17, Reference Torok, Ophel-Keller and Loo53) linking poorly performing and well-performing broilers to distinct GIT microbiome is a step in the right direction. It may be possible to evolve and develop FE to induce desirable changes in the gut microbiota for the enhancement of growth and uniformity of the production of commercial herds and flocks.
In studies involving microbial community analysis, the complexity of interpretation increases with the use of molecular techniques, all of which are subject to potential pitfalls of PCR and other limitations(Reference Marsh144, Reference Highlander145). No method is ideal, and even with the most advanced PCR-based techniques it is possible that microbial groups representing a significant fraction of the total microbial community escape detection. Consequently, it is possible that a link between microbiota and performance can be found using one method but not confirmed using some other method, even if the trial setup is identical(Reference Aphalajalati, Geier and Hughes141). Understanding the dynamics of the gut microbial community, or microbial balance, is necessary to establish or develop strategies to improve feed efficiency and growth rate, avoid intestinal diseases and proliferation of food-borne pathogens, and evolve efficacious FE systems. Undoubtedly, molecular microbiology techniques are an important strategy in such endeavours. The 16S rRNA gene sequences generated in various studies could be used as a basis for developing quantitative assays and thus allowing validation of the presence of performance-related gut bacteria; potentially, these assays could be used to monitor strategies to improve feed efficiency(Reference Torok, Hughes and Mikkelsen17). Such advances will probably be slow but ultimately optimal and detrimental microbial populations should be easily quantified and strategies to avert disease and poor performance more easily formulated(Reference Aphalajalati, Geier and Hughes141).
Conclusions
Although research on application of FE to modulate animal gut health is still in its infancy, the implications in terms of economic savings and its potential as an alternative technology to replace antibiotic growth promoters in pig and poultry feeding programmes are significant. Growing scientific evidence suggests that FE can be part of an integrated solution approach for containing enteric pathogens of economic importance. This value is not only captured in reduced medication costs, but also in a reduced variability in animal performance and reduced mortality by promoting gut health. Any additive, such as FE, which influences the digestibility of the diet will change the selection pressures on the microbiota which in turn will moderate the efficiency with which the host utilises its feed, and the immune interactions with the intestinal microbiome. However, the response of the microbiome to enzyme addition probably depends on the microbial status of the production system. Because FE are likely to change substrate characteristics (for example, release of xylo-oligosaccharides) and flow along the GIT, the subsequent microbiota responses will vary according to the populations present at the time of administration and their reaction to such changes, as well as the immune status of the animals and the presence of stress factors. It is not surprising that given the huge range of microbiota conditions likely to exist between studies, the responses to FE use, rather than being absolute, are a continuum or a population of responses. Nonetheless, recognition that FE can make an impact on the GIT microbiota and thus gut health will stimulate development of FE capable of modulating GIT microbiota to the benefit of host health under specific production conditions. The role of FE as part of an integral approach to animal health that is less reliant on antibiotic compounds appears to be important, although more research is needed to further elucidate factors affecting host–microbiome–diet interactions, and the strategies to alter those interactions in favour of the host.
Acknowledgements
E. K, L. F. R. and C. M. N. searched the literature, discussed and agreed on the review content, and wrote the paper; E. K. had primary responsibility for final content. All authors read and approved the final manuscript. This research did not receive specific grants from any funding agency in the public, commercial or not-for-profit sectors. E. K. and L. F. R. are employees of a feed additives supplier.
The authors do not have any conflict of interests.









