Age-related macular degeneration (AMD) is a major cause of severe irreversible visual impairment among elderly individuals in developed countries. It is estimated that approximately 8·7 % of persons over the age of 45 years worldwide were affected by this disease in 2014, and the number of persons with AMD will increase to 288 million by 2040(Reference Wong, Su and Li1). New therapies have been shown to effectively prevent progressive vision loss in certain types of exudative AMD; however, there remains a lack of an established effective therapy for most AMD patients, and it is difficult to restore the pathologically damaged retina to a healthy state with current therapies(Reference Mehta, Tufail and Daien2). Therefore, the identification of modifiable factors related to this disease is important to prevent the onset and progression of AMD.
Evidence from in vitro and animal experiments suggests that oxidative damage coupled with inflammation plays a vital role in the aetiology of AMD(Reference Abokyi, To and Lam3,Reference Liu, Gao and Cao4) . Accordingly, as they are important non-enzymatic anti-oxidative agents in the retina, vitamins and carotenoids were hypothesised to protect the retina from oxidative damage, which suggests that these nutrients play an important role in preventing the process of vision impairment(Reference Murugeswari, Firoz and Murali5,Reference Kamoshita, Toda and Osada6) . In particular, lutein and its structural isomer, zeaxanthin, which are concentrated in the macula, have also been suggested to play a role in protecting the macular region by acting as a blue light filter, thereby decreasing photochemical light damage(Reference Bernstein, Li and Vachali7).
To date, conflicting results have been reported on the association between the intakes of antioxidants, such as lutein, vitamin C and β-carotene and AMD, with some demonstrating a possible inverse association have been reported(Reference Nidhi, Mamatha and Padmaprabhu8–Reference Kim, Kim and Kwon13), whereas other studies showed no clear association(Reference Tan, Wang and Flood14–Reference Morris, Jacques and Chylack19). Moreover, the majority of these studies were conducted in the developed countries; to our knowledge, no data are available for the Chinese population. In addition, the evidence regarding the association between the predominant source of specific vitamins and carotenoids and AMD remains controversial(Reference Seddon, Ajani and Sperduto9,Reference Kim, Kim and Vijayakumar12,Reference Kim, Kim and Kwon13,Reference VandenLangenberg, Mares-Perlman and Klein16,Reference Cho, Seddon and Rosner18,Reference Moeller, Parekh and Tinker20,Reference Smith, Mitchell and Webb21) . Moreover, the co-consumption of various carotenoid-rich sources, such as raw vegetables, fruits and eggs, may potentially affect the absorption and bioavailability of carotenoids; however, few studies have examined the joint effects of the intakes of different sources of these nutrient on the risk of AMD.
Therefore, in this study, we investigated the association of vitamins and carotenoids, as well as their main dietary sources with the risk of AMD by conducting a population-based case–control study in the Chinese population.
Materials and methods
Participants
The Xi’an Eye Study is a multicenter, randomised, controlled, parallel group clinical trial designed to evaluate the effects of oral supplementation with macular xanthophylls alone or in combination with fish on the primary and secondary prevention of AMD (ChiCTR1900028680). Potential participants were recruited from clinics and health fairs between November 2016 and December 2019 by posting advertisements and flyers. A validated questionnaire was used to obtain detailed medical history, lifestyle, usual diet and other health-related information after obtaining written informed consent from each participant. Thorough ophthalmologic examinations, including the best-corrected visual acuity, intraocular pressure, slit lamp inspection, optical coherence tomography, fundus photography and fundus autofluorescence, were performed by ophthalmologists using a standardised protocol for all participants. Overnight blood samples were drawn at the same time as the dietary information was collected.
Participants aged ≥ 45 years with available data on dietary carotenoids and vitamins at baseline were included in the present investigation. Patients were eligible for inclusion in the analysis if they were first clinically diagnosed with early AMD (soft drusen and/or pigmentary abnormalities) or late AMD (geographic atrophy or signs of exudative AMD degeneration) by eye specialists according to the Age-Related Eye Disease Study classification system(22). Patients with a prior diagnosis of high myopia, glaucoma and clinically significant diabetic retinopathy or any other ocular disease that might affect central or parafoveal macular visual function were ineligible. In addition, those who had undergone recent cataract surgery or other intraocular surgery (within six months), followed special diets (such as vegetarians), had an unstable chronic illness or were taking photosensitising drugs (such as phenothiazines and chloroquine) were also excluded.
Using the same exclusion criteria, the same number of controls, who were matched for age and sex, were randomly selected during the same period from those without clinical signs of AMD. After applying the exclusion criteria, 260 controls and 260 cases remained for the current analysis.
The study adhered to the Declaration of Helsinki guidelines and was approved by the medical ethics committee of the Xi’an Jiaotong University Health Science Center. All participants provided written informed consent before participating in the study.
Dietary assessment
A validated semi-quantitative FFQ with eighty-six items was used to collect the dietary information. Participants were asked about the portion size and how many times, on average, they had consumed each food throughout the previous year, with five possible responses (per day, week, month, year or never). A colour food photography atlas with different portion sizes for all food items was used to produce a more accurate estimate. Nutrient and energy intakes were calculated by converting the frequency of consumption and portion size estimates to daily intake, then multiplying the daily intake with the corresponding nutrient contribution of a standard portion (100 g) for each item, summed across all foods consumed. The nutrient composition values were primarily based on the food composition tables from the China Food Composition Database and other published sources (such as scientific literature from local researchers)(Reference Yang, Wang and Pan23).
The FFQ used in the present analysis was validated to three consecutive 24-h dietary recalls in a subsample of participants (n 31). The value of the Spearman correlation coefficient was 0·37 (P = 0·05) for lutein, 0·42 (P = 0·02) for α-carotene, 0·32 (P = 0·07) for β-carotene, 0·34 (P = 0·06) for β-cryptoxanthin, 0·79 (P < 0·001) for retinol and 0·36 (P = 0·05) for vitamin C.
Assessment of covariates
We collected information on medical, lifestyle and other health-related factors, such as body weight, socioeconomic status, smoking status, alcohol consumption, sunlight exposure, physical activity, medication or supplement use, family history of major chronic conditions and personal history of chronic diseases. BMI was calculated as the ratio of weight in kilograms to the square of the height in metres.
Statistical analysis
For those with questionnaires with missing information for individual food data when aggregating items to calculate the composite items, we assumed that they did not consume that food(Reference Joshipura, Ascherio and Manson24). Participants with incomplete dietary data (blank responses for more than ten items) or implausible reported dietary assessments (i.e. < 800 or > 6000 kcal/d for men and < 600 or > 4000 kcal/d for women) were excluded. Exposure variables were adjusted for total energy intake using the density method (intake per 1000 kcal). The participants were divided into quartiles according to the average vitamin or carotenoid intake, with the lowest quantile treated as the reference group. The category-specific OR and 95 % CI were estimated using conditional logistic regression analysis. In the multivariate analysis, we adjusted for smoking status (never, past or current smoker), educational level (less than college or college), regular physical activity (yes or no), total energy intake (continuous), blood cholesterol (continuous), HDL-cholesterol (continuous) and LDL-cholesterol (continuous). Trend analyses across increasing quartiles of nutrient intake were conducted by assigning a median value for each dietary exposure variable category, which was treated as a continuous variable in the regression models. In addition, restricted cubic spline regression with three knots was used to assess the non-linear dose–response relationship.
The primary exposures for this analysis were lutein, α-carotene, β-carotene, β-cryptoxanthin, retinol and vitamin C. Because significant results were observed in the primary analysis of xanthophyll, we further explored the consumption of foods rich in lutein to provide more insight into the primary results. In addition, we evaluated the joint associations of different vitamin- and carotenoid-rich foods (three categories) with the AMD risk. All statistical analyses were performed using Stata 12.0 (StataCorp), and a two-sided P < 0·05 was considered statistically significant.
Results
In total, 260 AMD cases and an equal number of disease-free controls were included in the current analysis (Fig. 1). The characteristics of the patients with AMD and controls included in this study are presented in Table 1. Compared with non-AMD controls, the AMD patients tended to have a lower educational status, were more likely to smoke and drink alcohol and were less likely to engage in regular exercise.
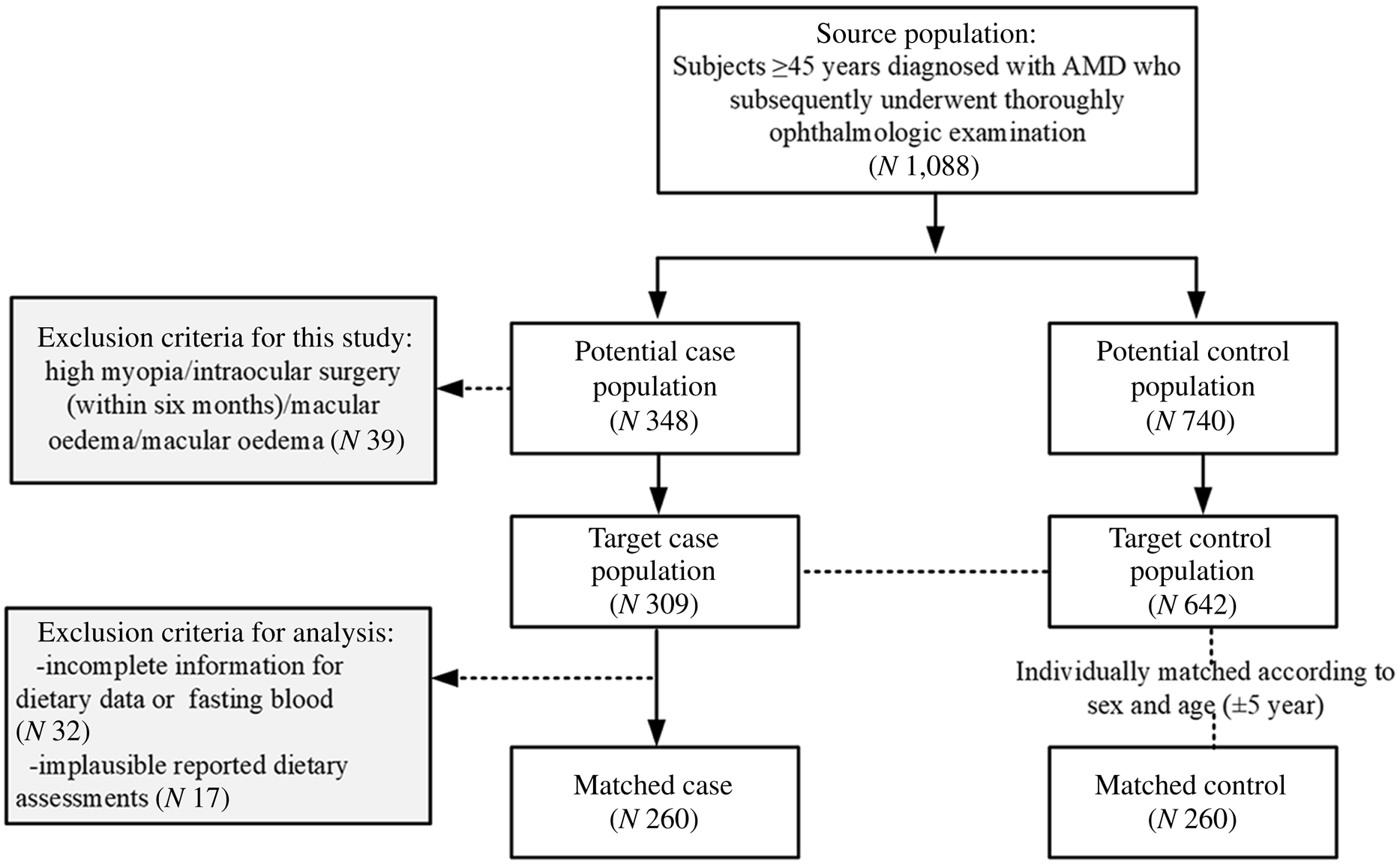
Fig. 1. Flow diagram of selection of the study sample in the matched case–control study.
Table 1. Baseline characteristics of the study sample*
(Number and percentages; mean values and standard deviations)
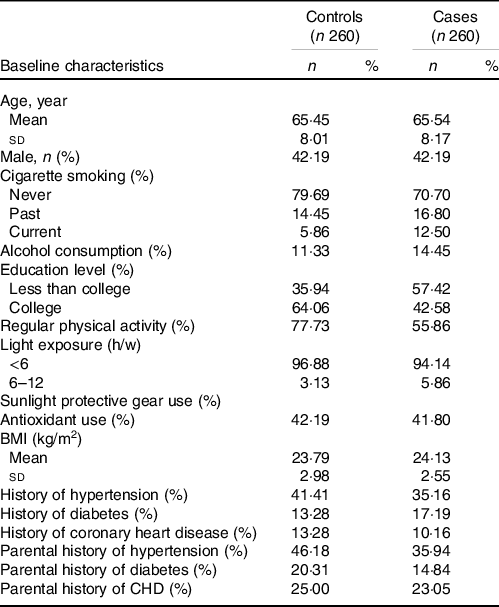
* Categorical variables are expressed as n (%); the continuous variables are expressed as the mean sd values.
Table 2 exhibits the associations between dietary vitamin and carotenoid intake and AMD risk. In the age- and sex-adjusted model, lutein intake was associated with a lower AMD risk. After adjusting for confounding variables in the multivariate model, an increasing lutein intake was inversely associated with the AMD risk, and the OR (95 %CI) across quartiles of dietary lutein intake were 1·0, 0·44 (95 % CI: 0·18, 1·09), 0·39 (95 % CI: 0·15, 1·00) and 0·30 (95 % CI: 0·10, 0·88) (P trend = 0·04), respectively. A similar inverse association with AMD risk was observed for β-cryptoxanthin, with a multivariate adjusted OR (95 % CI) for the highest quartile of intake compared with the lowest quartile of 0·28 (95 %CI: 0·11, 0·74; P trend = 0·02). In contrast, weak or null associations with AMD were observed for the dietary intake of other antioxidant nutrients. The risk of AMD decreased rapidly with increasing dietary lutein intake until approximately-2000 μg/1000 kcal per day (P for non-linearity < 0·01; online Supplemental Fig. 1). Likewise, a nonlinear association was also observed for β-cryptoxanthin in the spline analysis (P for non-linearity < 0·01; online Supplemental Fig. 2).
Table 2. Odds ratio (95 % confidence intervals) of age-related macular degeneration according to quartiles of nutrient intake
(Odd ratio and 95 % confidence intervals)

* Reference group.
† P for trend over the quintile categories used the median for each quintile category as a continuous variable.
‡ Model 1: no adjustment.
§ Model 2: adjusted for smoking status, educational level, regular physical activity, dietary total energy intake, blood cholesterol, HDL-cholesterol and LDL-cholesterol.
Given the vital role of lutein in maintaining a normal morphology and function of the retina and the strength of the association between this carotenoid and AMD risk, we also examined the risk of AMD by major food sources of lutein, including spinach, rapeseed, broccoli, pumpkin, carrot, sweet potatoes, citrus fruits and eggs (Table 3). Compared with the participants who consumed < 1 servings of spinach per week, those who consumed ≥ 2 servings of spinach per week had a 58 % (95 % CI: 12 %, 80 %; P trend = 0·03) lower risk of AMD. In contrast to spinach, the inverse association between the intakes of other lutein-rich vegetables and AMD was weak and non-significant. When compared with the participants who ate ≤ 2 servings of egg per week, the multivariable pooled OR (95 % CI) of AMD was 0·52 (95 % CI:0·27, 0·98) for those with an intake of ≥ 1 serving egg per day (P trend = 0·07). We observed a monotonically decreasing risk of AMD associated with increasing egg intake (P for non-linearity = 0·06, online Supplemental Fig. 3).
Table 3. Odds ratio (95 % confidence intervals) of age-related macular degeneration by frequency of consumption of foods rich in carotenoids
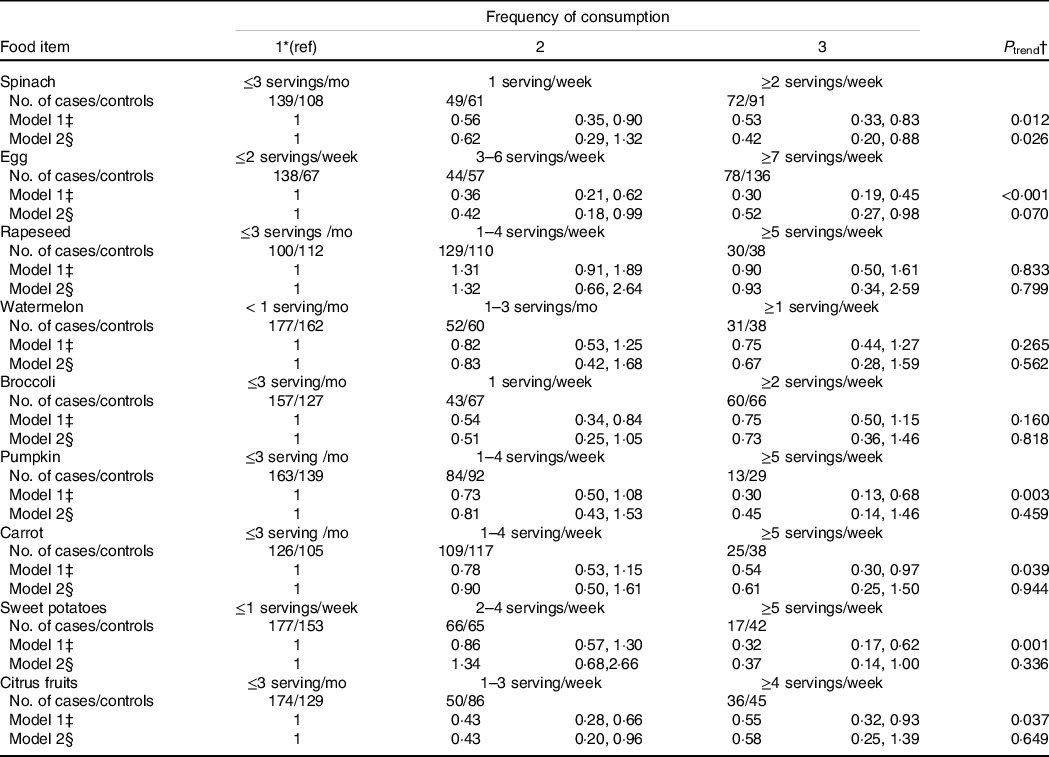
* Reference group.
† P for trend over the quintile categories used the median for each quintile category as a continuous variable.
‡ Model 1: no adjustment.
§ Model 2: adjusted for smoking status, educational level, regular physical activity, dietary total energy intake, blood cholesterol, HDL-cholesterol and LDL-cholesterol.
We further examined the joint association of egg and spinach intake with the risk of AMD. Participants who were in the highest category of both egg and spinach intake had the greatest reduction in AMD risk in comparison with those who were in the lowest category of consumption, and the OR was 0·23 (95 % CI: 0·08, 0·71; Fig. 2).
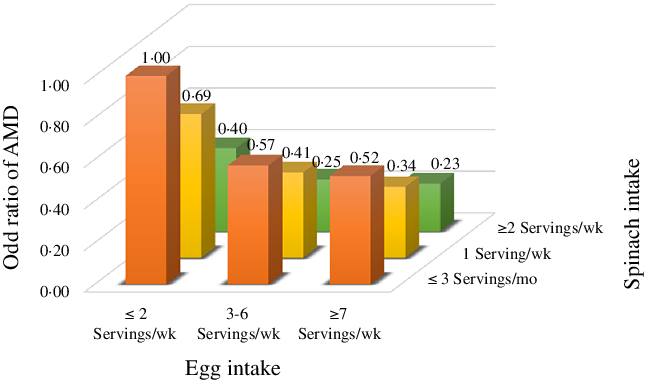
Fig. 2. Joint association of spinach and egg intake with risk of age-related macular degeneration. OR were calculated in conditional logistic models after adjusting for smoking status, educational level, regular physical activity, dietary total energy intake, blood cholesterol, HDL-cholesterol and LDL-cholesterol.
Discussion
In the present study, a higher intake of dietary lutein and β-cryptoxanthin was associated with a lower risk of AMD. The consumption of lutein-rich foods, such as spinach or eggs, was also inversely associated with the risk of AMD. The decrease in the risk of AMD was more pronounced among participants who had a higher combined consumption of spinach and eggs. These findings provide further evidence for the benefits of lutein and lutein-rich foods in preventing of AMD.
To date, observational data related to specific dietary vitamin and carotenoid intake and AMD risk have been predominantly analyzed in Western populations. In the Nurses’ Health Study and the Health Professionals Follow-up Study, Wu et al. reported that individuals with a higher intake of bioavailable lutein, β-cryptoxanthin, and α-carotene were had an approximately 30 %–40 % lower risk of advanced AMD(Reference Wu, Cho and Willett10). Similarly, in a population of 4519 men and women aged 60 to 80 years in the Age-Related Eye Disease Study, SanGiovanni et al. observed that lutein intake was inversely associated with the risk of advanced AMD and large or extensive intermediate drusen(22). Our previous meta-analyses, which were mainly based on studies carried out in Western populations, also suggested that a high dietary intake of lutein yielded a 32 % reduction in the risk of late AMD(Reference Ma, Dou and Wu25). In agreement with these findings, our results suggest that increasing the intakes of vitamins and carotenoids, especially lutein, was inversely associated with the risk of AMD. In addition, a similar inverse association between spinach consumption and lutein intake observed in our study supports the hypothesis that lutein in spinach may be responsible for the potential beneficial effect of spinach on AMD risk.
Several potential mechanisms for specific antioxidants constituents reducing biological pathways related to AMD development have been suggested, including the quenching of singlet oxygen, inhibition of lipid peroxidation and reduction of the inflammatory response(Reference Ozawa26). The retina is particularly susceptible to oxidative damage due to its continuous intense exposure to short-wavelength visible light, in combination with its high concentration of oxygen and PUFA(Reference Tao, Zhou and Zhu27). The uncontrolled oxidative injury could contribute to lipofuscin formation and induce a chain reaction of lipid peroxidation(Reference Maeda, Maeda and Golczak28). In addition to disruption of the structure of retina stability and integrity directly, the accumulation of lipofuscin could increase the susceptibility of retina components to phototoxicity, which has been implicated in the pathophysiology of AMD(Reference Fanjul-Moles and López-Riquelme29). Vitamins and carotenoids can attenuate photo-oxidative injury through the scavenging of reactive oxygen species, thereby counteracting the process of complement activation and inflammation(Reference Li, Fu and Lo30). In vitro studies have also revealed that ARPE-19 cells with carotenoid supplementation exhibited increased viability and less accumulation of lipid hydroperoxides, indicating the importance of the carotenoids in the efficient protection against oxidative damage induced by photosensitised reactions(Reference Wrona, Rózanowska and Sarna31). Vitamins and carotenoids may be related to the capacity to modulate the expression of inflammation-related genes involved in chronic local inflammatory responses within the retina, which are believed to play a critical role in the pathophysiology of drusen(Reference Bian, Gao and Zhou32,Reference SanGiovanni and Neuringer33) . Using a murine model of laser-induced choroidal neovascularisation, lutein supplementation led to substantial inhibition of macrophage infiltration into the retina choroidal neovascularisation area by suppressing tumour necrosis factor α-induced nuclear factor (NF)-kB activation and NF-kappaB p65 nuclear translocation in vivo and in vitro (Reference SanGiovanni and Neuringer33). Leung et al. observed that carotenoids exhibited benefits in reducing the non-enzymatic oxidation of n-6 PUFA and appeared to regulate inflammatory lipid mediators(Reference Leung, Galano and Crauste34). In addition, the macular xanthophylls were uniquely concentrated at the macula, and the absorbance spectrum peak of these macular pigment coincides with the absorbance spectrum of short-wavelength visible light, which protects the macular region by filtering damaging blue light, thereby possibly attenuating photochemical light damage(Reference Barker, Snodderly and Johnson35).
It should be noted that the inverse association was suggested to be stronger for the additive intakes of spinach and egg in the joint analysis. Although eggs contain markedly less lutein than dark-green leafy vegetables, lutein bioavailability from eggs is higher than that from other food sources, possibly owing to the presence of the lipid matrix in egg yolk(Reference Battaglia Parodi, Brunoro and Tomasso36). In a crossover trial, Chung et al. observed that lutein-enriched egg consumption led to the highest serum lutein response compared with lutein supplements and spinach(Reference Chung, Rasmussen and Johnson37). In addition, the influence of egg consumption on the incidence of AMD may be potentially mediated by other nutrients and compounds in the diet.
The egg yolk matrix, which has a high lipoprotein content, could increase the bioaccessibility and bioavailability of lutein(Reference Kelly, Nolan and Howard38). It has been indicated that co-consuming eggs could enhance the absorption of total carotenoids from other carotenoid-rich foods such as raw vegetables(Reference Kim, Gordon and Ferruzzi39). From a public health prospective, as eggs are a low-cost, widely available and easily digestible source of many nutrients, it would feasible to encourage a moderate egg consumption, as a factor in the context of a healthy diet that can help to prevent AMD.
Our results must be interpreted within the context of several limitations. First, given the nature of case–control studies, our results cannot establish causality between the intakes of carotenoids and vitamins and the risk of AMD, as the possibility of unmeasured or residual confounding cannot be ruled out and these confounders could theoretically affect the observed associations. However, several established and potential risk factors for AMD were statistically controlled for the present study, which may minimise the potential impact of residual confounding to some extent. Second, although the FFQ used in the present analysis was validated against dietary records, the assessment of diet was still inevitably prone to measurement error. Moreover, individuals’ eating patterns and food composition levels may change over time, so a single measurement may not fully represent the variability of carotenoids and vitamins in the development of AMD, which has a long aetiologic period. However, in addition to a reasonably high correlation between repeated measures on the same samples over a one-year period, individuals are less likely to switch their habitual diet or lifestyle as a result of the diagnosis because the relatively high quality of vision may mask the effect of AMD for many years. Third, nutrients in the diet may have additive or synergistic effects on the occurrence of AMD; therefore, we cannot rule out the potential synergistic effects of lutein and other dietary factors on AMD risk. In addition, detailed information regarding food storage conditions and cooking methods were not collected by the present FFQ, and this may introduce additional measurement errors. Therefore, the associations between circulating biomarker status and AMD risk among the same population should be considered in future research before considering the possible implications for public health. Finally, although the participants in the present study were recruited from different centers, the study population was only composed of residents with Chinese ancestry, which may reduce the generalisability of our results to the non-Chinese population.
In conclusion, the results of this study indicate that the dietary intake of vitamins and carotenoids, especially lutein, as well as its major food sources, is significantly and inversely associated with AMD risk, which supports the dietary recommendations to increase consumption of lutein-rich foods to facilitate the prevention of AMD. Along with our findings on the importance of increasing the bioaccessibility and bioavailability of lutein, the joint beneficial effects of different lutein-rich foods on the risk of AMD warrant further investigation in future studies.
Acknowledgements
This study was partially supported by grants from the National Natural Science Foundation of China (NSFC-82022062; NSFC-81973025); Nutrition Science Research Foundation of BY-HEALTH (TY0181101); Key Research and Development Program of Shaanxi (2022SF-185) and the Fundamental Research Funds for the Central Universities (qngz2016004, xzy032019008). The funders had no role in the study design, implementation, analysis, decision to publish or reparation of the manuscript.
The authors’ contributions are as follows: H. J., L. N. W., D. L. W., C. P., C. L., B. B. M., F. Y. C. and L. M. contributed to the study design and data analyses; H. J., S. J. L., B. Y. L., Y. H. F., M. M. and Z. F. L. was the principal investigator and contributed to the study design and interpretation of the findings and wrote the manuscript; J. S., J. L. and C. L. contributed to the study design and performed the data analyses. All authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522002161








