Iodine is necessary for the synthesis of thyroid hormones, such as triiodothyronine and tetraiodothyronine, which are essential for all aspects of human metabolism(Reference Watutantrige Fernando, Cavedon and Nacamulli1,Reference Mansourian2) . Chronic iodine deficiency can lead to iodine deficiency disorders, which may adversely affect the physical and mental development of people of all ages(Reference Rochau, Qerimi Rushaj and Schaffner3). Iodised salt has been introduced in many countries, including Switzerland, Sweden, Croatia, Italy, Spain, Mongolia and China, as a safe, cost-effective and sustainable way to eliminate iodine deficiency disorders(Reference Nystrom, Brantsaeter and Erlund4–Reference Tran, Hetzel and Fisher6). Shanghai, which is the biggest coastal city with low water iodine in China, has followed the national compulsory universal salt iodisation (USI) policy since 1996.
The most vulnerable group in terms of iodine deficiency disorders is that of pregnant women, due to the increased need for iodine during the growth of the fetus as well as the increased blood volume and urine excretion. The most damaging effects of iodine deficiency disorders in pregnancy are poor physical growth and brain development of the fetus and possible abortion, stillbirth, birth defects, perinatal mortality and endemic cretinism(Reference Yang, Li and Liu7,Reference Moreno-Reyes, Glinoer and Van Oyen8) . To maintain an appropriate iodine level, Shanghai has monitored household cooking salt iodine concentration (SIC) since 1995. The SIC standard has been adjusted three times in Shanghai: 20–60 mg/kg in 1996, 20–50 mg/kg in 2000 and 21–39 mg/kg in 2012. The median urine iodine concentration (UIC) of 8–10-year-old children in Shanghai has been in an appropriate range since 2005, which indicates adequate iodine intake. However, the median UIC of pregnant women was 139·8 µg/l in 2011 and 126·5 µg/l in 2015; both are slightly below the adequate median UIC range of 150–249 µg/l as recommended by the WHO/UNICEF/International Council for Control of Iodine Deficiency Disorders (ICCIDD)(Reference Wang, Liu and Su9,10) . In 2009, a cross-sectional survey of all districts in Shanghai showed that the median iodine concentration in drinking water, which at 12·8 µg/l, contributed just 10·0 % of the total iodine. The remainder came from the diet, of which iodised salt contributed 63·5 %(Reference Zou, Wu and Guo11).
Monitoring data in Shanghai showed that the percentage of households choosing iodised salt dropped from 94·6 % in 1999 to 76·5 % in 2017(Reference Wang, Zang and Shi12). In theory, with more families opting for non-iodised salt, the median UIC in the population should be on a downwards trend. However, our monitoring showed little fluctuation, which may be related to the high rate of eating out in Shanghai. The USI policy stipulated that all centralised meal units (including restaurants and canteens) must use iodised salt. With the development of the economy, eating out is becoming more common in Shanghai: the Shanghai Diet and Health Survey showed that the proportion of people who eat at least one meal prepared away from home in the past week was 55·1 %(Reference Zang, Luo and Wang13). Therefore, we investigated the effect of household SIC and eating out on UIC in pregnant women, to guide residents to choose household cooking salt and iodine supplements correctly and rationally as well as to formulate relevant indicators for policy evaluation.
Materials and methods
Population and study design
The data were taken from a cohort study (April to October 2017) that investigated the effects of iodine levels in pregnant women on the growth and development of their offspring. The target population was pregnant women who had lived in Shanghai for at least 6 months over the past year with no cognitive and activity impairment, no infectious disease and cooperation with the investigation. The formula for calculating complex sampling sample size which was ![]() $n = {\rm{ }}{\left( {{{{\mu _{\alpha /2}} \times \sigma } \over \delta }} \right)^2} \times {\rm{deff}}$ was used to calculate the sample size required for analysis(Reference Yan14). According to the results of pregnant women iodine nutrition survey in 2009 in Shanghai, σ = 106·83 μg/l, δ = 15 μg/l. We defined the two-sided significance level α = 0·05, 1–β = 0·8, µ α/2 = 1·96. The deff value of complex sampling was 2. There were nine levels by gestational weeks (first trimester, second trimester and third trimester) and the age of pregnant women (<30, 30–35, >35 years old), at least 3510 pregnant women were needed for analysis. Considering 20 % would be lost to follow-up and 20 % would refuse to visit, a total of 5·485 pregnant women were recruited. A multistage, stratified random sampling method was used to obtain a representative sample. The number surveyed in each administrative district was determined according to the required sample size and the number of pregnant women in each district in 2016. Each district was divided into five sections, from which a street was randomly selected. An equal number of pregnant women were selected from each section and different gestational weeks were evenly distributed.
$n = {\rm{ }}{\left( {{{{\mu _{\alpha /2}} \times \sigma } \over \delta }} \right)^2} \times {\rm{deff}}$ was used to calculate the sample size required for analysis(Reference Yan14). According to the results of pregnant women iodine nutrition survey in 2009 in Shanghai, σ = 106·83 μg/l, δ = 15 μg/l. We defined the two-sided significance level α = 0·05, 1–β = 0·8, µ α/2 = 1·96. The deff value of complex sampling was 2. There were nine levels by gestational weeks (first trimester, second trimester and third trimester) and the age of pregnant women (<30, 30–35, >35 years old), at least 3510 pregnant women were needed for analysis. Considering 20 % would be lost to follow-up and 20 % would refuse to visit, a total of 5·485 pregnant women were recruited. A multistage, stratified random sampling method was used to obtain a representative sample. The number surveyed in each administrative district was determined according to the required sample size and the number of pregnant women in each district in 2016. Each district was divided into five sections, from which a street was randomly selected. An equal number of pregnant women were selected from each section and different gestational weeks were evenly distributed.
The medical ethics committee of the Shanghai Municipal Center for Disease Control and Prevention (CDC) approved the study. Written informed consent was given by all participants.
Household condiment weighing and questionnaire survey
A household condiment weighing method was used to collect data on cooking oil, salt and soya sauce by weighing changes in the condiment inventory over a continuous period of 1 week. At the same time, the number of people who consumed the household condiments at each meal was recorded, and a household salt sample was collected for iodine concentration detection. Participants were required to complete a standardised questionnaire, which included demographic information and food consumption, through face-to-face interviews with trained and evaluate qualified investigators. All data were reviewed by the local district CDC project team, and at least 5 % of data were reviewed by the Shanghai CDC project team. All flawed questionnaires were reexamined until there were no defects. Participants who were unable to complete the survey were not included in the final analysis.
Household cooking salt and urine sample collection and analyses
A 60 g sample of cooking salt was collected from each household of subject during the condiment weighing week, and a 50 ml urine sample was collected from each subject at the end of the week. Household SIC and UIC were measured by titration and acid digestion, respectively(15,16) , at the Central Laboratory of Shanghai CDC and sixteen District CDC in Shanghai, all of these laboratories which must past the assessment of the National Iodine Deficiency Disorders Reference Laboratory of China CDC before carrying out the test. The internal quality-control samples for the analysis of SIC and UIC were provided by China CDC.
Definition and classification of relevant indicators
Salt was divided into three types according to the SIC standard in Shanghai: (1) non-iodised salt with an iodine content <5 mg/kg; (2) low-iodine salt, 5 mg/kg ≤ iodine content < 21·0 mg/kg and (3) qualified-iodised salt with an iodine content ≥ 21·0 mg/kg. The iodised salt usage rate is the amount consumed with an iodine content ≥ 5·0 mg/kg in all samples. The qualified-iodised salt usage rate is the amount consumed with an iodine content ≥ 21·0 mg/kg in all samples.
Frequency of eating out was divided into three groups: never (not eaten out in the past week), occasionally (eating out less than once/d in the past week) and frequently (eating out at least once a day in the past week).
The iodine nutritional status of pregnant women was determined according to the criteria recommended by the WHO/UNICEF/ICCIDD. Insufficient iodine intake was defined as median urinary iodine (MUI) < 150 µg/l; adequate iodine intake as MUI 150–249 µg/l; iodine intake above the requirement as MUI 250–499 µg/l and excessive iodine intake as MUI ≥ 500 µg/l(10).
Statistical analysis
Data processing and statistical analyses were conducted with EpiData (version 2.0, The EpiData Association), Excel (2010 edition, Microsoft) and SPSS (version 22.0). Normally distributed data were expressed as the mean values and standard deviations, and non-parametric data were expressed as the median (25th percentile, 75th percentile). A one-way ANOVA was used to compare multiple groups. In pairwise comparisons, the homogeneity of variance was tested by the least significant difference t test (LSD) and the inhomogeneity of variance was assessed by Tamhane’s T2 test. A Kruskal–Wallis one-way ANOVA test (K) was used to compare non-parametric data across multiple groups. A P value of < 0·05 was considered significant.
Results
General characteristics of study participants were compared
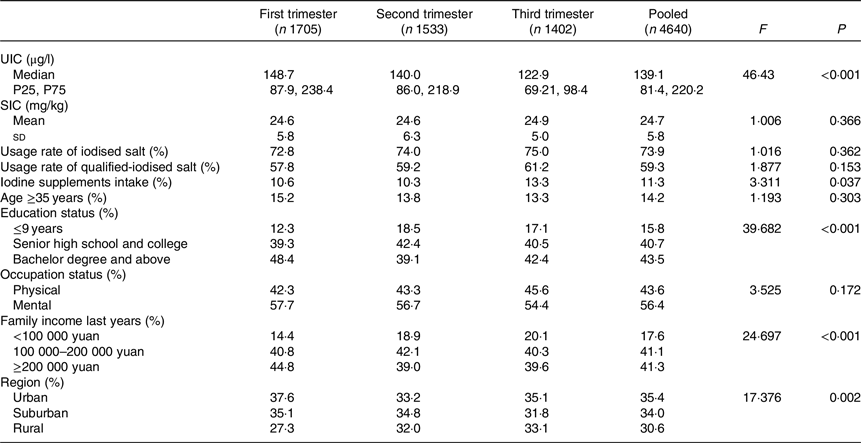
A total of 4640 valid subjects were investigated. The median UIC was 139·1 μg/l in all subjects and 148·7, 140·0 and 122·9 μg/l in women in the first, second and third trimesters, respectively. There were significant (P < 0·001) differences in median UIC among the different gestational weeks (first trimester, second trimester and third trimester). Pairwise comparisons showed that the adjusted P values were <0·001 between the third and first trimesters, 0·004 between the third and second trimesters and 0·159 between the second and first trimesters. Median UIC was higher in the first and second trimesters than in the third trimester.
A total of 3427 households ate iodised salt, of which 2750 were qualified-iodised salt. The usage rates of iodised salt and qualified-iodised salt were 73·9 and 59·3 %, respectively. The average iodine content in iodised salt was 24·7 mg/kg. There were significant differences in iodine supplement intake, education status, family income in the previous year and region between different gestational weeks with P values of 0·037, <0·001, <0·001 and 0·002, respectively (Table 1). There were no statistically significant differences between any other indexes.
Table 1. Characteristics of study participants stratified by gestational week
(Mean values and standard deviations; median values and 25th, 75th percentiles (P25, P75); percentages)
UIC, urinary iodine concentration; SIC, salt iodine concentration.
The distribution of urinary iodine concentration and frequency of eating out
The percentages of UIC which ranged from 150 and 250 μg/l were 26·7, 27·9 and 24·2 % in the first, second and third trimesters, respectively (Fig. 1).
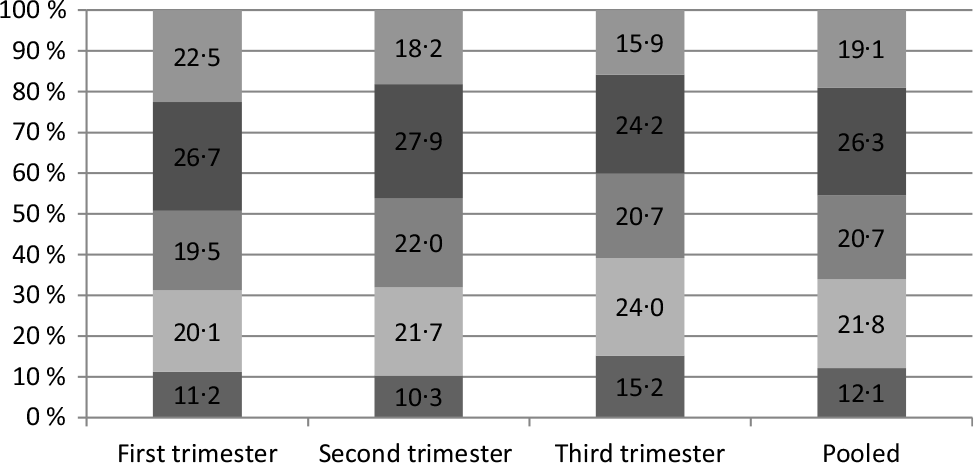
Fig. 1. Proportions of different urinary iodine concentration rates in pregnant women (%). ![]() , >250 µg/l;
, >250 µg/l; ![]() , 150–250 µg/l;
, 150–250 µg/l; ![]() , 100–150 µg/l;
, 100–150 µg/l; ![]() , 50–100 µg/l;
, 50–100 µg/l; ![]() , <50 µg/l.
, <50 µg/l.
Pregnant women (40·2 %) had never eaten out in the past week, 37·7 % had eaten out less than once a day and 22·1 % had eaten out greater than or equal to once a day (Fig. 2). Analysis of the constituent ratio of eating out frequencies among different gestational weeks showed that the difference was statistically significant (P < 0·001).
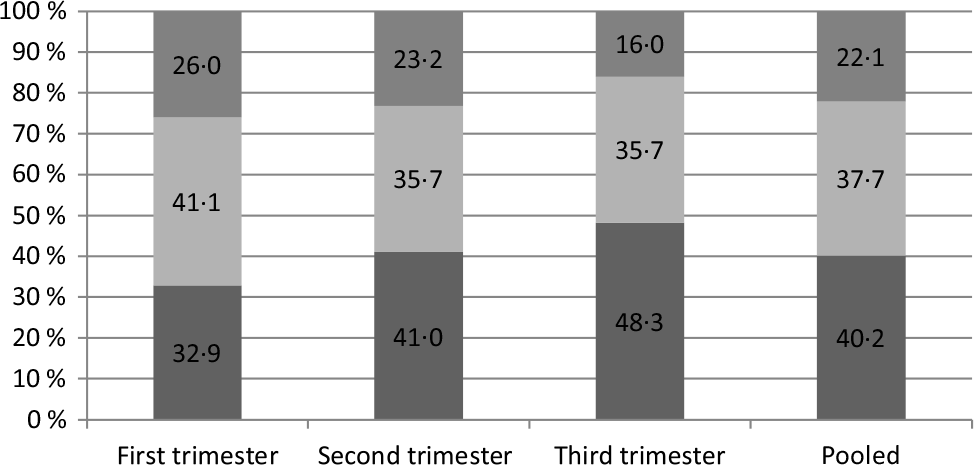
Fig. 2. Frequency of eating out in the past week for pregnant women (%). ![]() , ≥1 time/d;
, ≥1 time/d; ![]() , <1 time/d;
, <1 time/d; ![]() , 0 times.
, 0 times.
Comparison of urinary iodine concentration among different cooking salt iodine concentration groups and eating out frequency groups
Among the different SIC groups, there were significant differences in median UIC only in pooled subjects (F = 6·098, P = 0·047, Table 2). Pairwise comparisons performed between the three groups showed that the adjusted F and P were 1·102 and 0·941 between the non-iodised salt group and the low-iodine salt group, 3·399 and 0·037 between the non-iodised salt group and qualified-iodised salt group and 1·005 and 1·000 between the low-iodine salt group and qualified-iodised salt group. The median UIC was higher in the qualified-iodised salt group than in the non-iodised salt group. There were no statistically significant differences in UIC between frequencies of eating out.
Table 2. Urinary iodine concentration (μg/l) of pregnant women between different cooking salt iodine content groups and eating out frequency groups
(Median values and 25th, 75th percentiles (P25, P75))
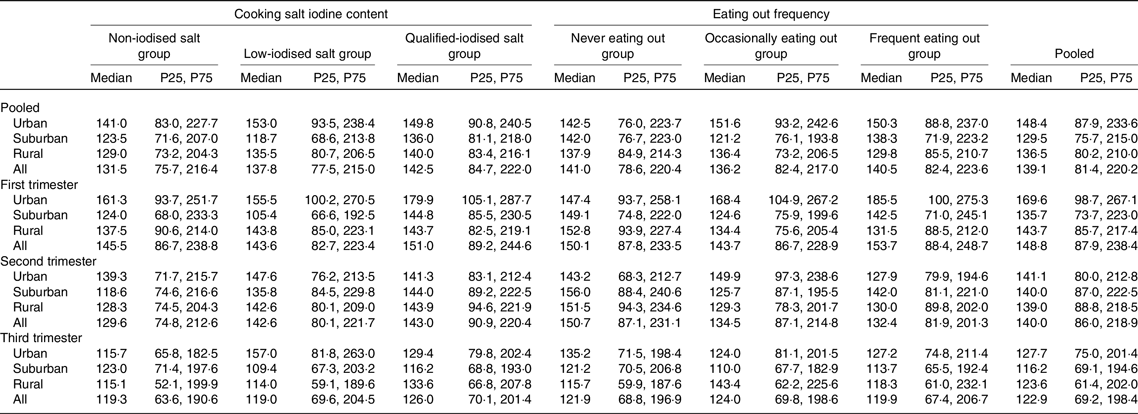
There were significant differences in median UIC among different regions only in the first trimester and in pooled subjects (F = 31·186, P < 0·001 and F = 15·720, P < 0·001). The adjusted F and P were 0·774, 1·000 and <0·001, 1·000 between suburban and rural subjects, 5·234, <0·001 and 3·359, 0·002 between suburban and urban subjects and 4·097, <0·001 and 3·453, 0·002 between rural and urban subjects, respectively. The median UIC was higher in urban subjects than in suburban and rural subjects.
Combined effect of household cooking salt iodine concentration and eating out on urinary iodine concentration
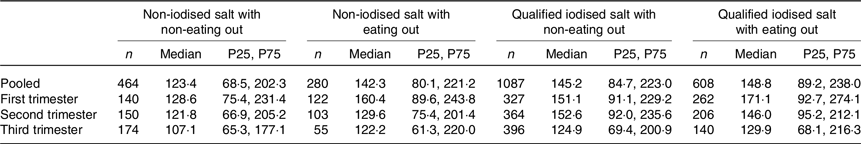
The results from the iodised/non-iodised salt groups were combined with the results of eating out/not eating out to form four groups for discuss the combined effect of these two variables: (1) non-iodised salt and not eating out (464 women); (2) non-iodised salt and eaten out at least once a day in the past week (280 women); (3) qualified-iodised salt and not eating out (1087 women) and (4) qualified-iodised salt with eating out (608 women). The median UIC were 123·4, 142·3, 145·2 and 148·8 μg/l, respectively (Table 3). There were significant differences in median UIC only in pooled subjects (F = 17·332, P = 0·001). The adjusted F and P of the pairwise comparisons were 2·337 and 0·117 between groups 1 and 2, 3·265 and 0·007 between groups 1 and 3, 4·055 and <0·001 between groups 1 and 4, 0·063 and 1·000 between groups 2 and 3, 1·012 and 1·000 between groups 2 and 4 and 1·361 and 1·000 between groups 3 and 4. The results showed that median UIC was lower in the non-iodised salt with non-eating out group than in the qualified-iodised salt groups under both conditions of eating out.
Table 3. Combined effect of household cooking salt iodine concentration and eating out frequency on urinary iodine concentration (μg/l)
(Median values and 25th, 75th percentiles (P25, P75))
Discussion
The quality of nutrition during pregnancy affects not only the health of the mother and fetus but also the health of the baby after birth. Although numerous studies have shown a U-shaped relationship between iodine and human health(Reference Bath17,Reference Laurberg, Bulow Pedersen and Knudsen18) , the most common health problems are associated with iodine deficiency, especially in iodine-deficient areas(Reference Song, Zou and Guo19). Iodine deficiency during pregnancy is an important global public health issue and the leading preventable cause of neural and physical developmental impairments worldwide(Reference Nazeri, Shab-Bidar and Pearce20). In our survey, the women were mildly iodine deficient compared to the range recommended by international standards(10,Reference Nazarpour, Ramezani Tehrani and Behboudi-Gandevani21) . The proportion of pregnant women taking an iodine-containing supplement was similar to previous studies in Switzerland(Reference Zimmermann, Aeberli and Torresani22,Reference Andersson, Aeberli and Wust23) .
A previous study compared UIC in women during and after pregnancy and found that in an iodine sufficient area, iodine excretion was lower during than after pregnancy. However, the current standard during pregnancy recommended by WHO/UNICEF/ICCIDD is based on non-pregnant populations: this might lead to overestimation of iodine deficiency during gestation, so the lower limit may be too high(Reference Castilla, Murcia and Arrizabalaga24). Monitoring by the Shanghai CDC showed that the median UIC in children aged 8–10 years was >100 μg/l after the implementation of the USI policy, indicating that children aged 8–10 years have sufficient iodine intake(Reference Wang, Zang and Shi12). Since the median UIC from a representative child’s spot urine sample can be used to classify the iodine status of a population(Reference Melse-Boonstra and Jaiswal25). These suggest that the general population in Shanghai has been in an iodine sufficient status since the implementation of the USI policy. Based on the above analysis, we find that the median UIC in the first and second trimesters of pregnancy are basically sufficient to meet the needs of the mother and fetus, although they are slightly below the international standards. However, the UIC in the third trimester of pregnancy was obvious low. These results are consistent with previous studies in Australia and Sri Lanka, both of which suggest that women in the third trimester of pregnancy have a higher risk of iodine deficiency(Reference Stilwell, Reynolds and Parameswaran26,Reference De Zoysa, Hettiarachchi and Liyanage27) . In Shanghai, it is therefore necessary to remind pregnant women in the third trimester to take an iodine supplement or to choose iodine-rich food.
Our study found that the median UIC of the qualified-iodised salt group was higher than in the non-iodised group. The difference was statistically significant, but the practical significance of the gap is limited. Based on a previous study, salt iodine contributed 63·5 % of dietary iodine intake in Shanghai and so this gap would be wider if all iodine intake came from home cooking salt(Reference Zou, Wu and Guo11). This difference between the salt groups may be related to the increasing frequency of eating out, which is also an important opportunity for iodised salt intake. With changes in demographic and socio-economic characteristics, eating out is becoming more popular around the world and generally involves consuming more food, including salt(Reference Kim and Ahn28–Reference Paddock, Warde and Whillans30). According to the USI policy, all centralised meal units (including restaurants and canteens) must use iodised salt in non-high iodine areas. As an iodine-deficient coastal area, centralised meal units in Shanghai use iodised salt. This study found that 40·2 % of pregnant women had not eaten out in the past week, 37·7 % had eaten out less than once a day and 22·1 % had eaten out at least once a day. In addition, women were more likely to eat at home by the third trimester, which may be related to the Chinese government’s maternity leave policy. On the other hand, it may be related to the reduced physical and social activities of pregnant women in the third trimester.
Our study found no statistically significant difference between the different frequencies of eating out. Considering the possible interaction between eating out and SIC, we analysed the two variables together and divided the population into four groups. The results showed that the median UIC of women who use non-iodised salt and did not eat out was lower than those who used qualified-iodised salt and ate out at least once a day. Even when there was no significant difference (between non-iodised salt with non-eating out and non-iodised salt with eating out), the median UIC absolute difference was 18·9 μg/l. This further shows the importance of iodised salt on the iodine intake of pregnant women in Shanghai. Especially in the third trimester, if families use non-iodised salt and the pregnant woman do not eat out, the median UIC was just 107·1 μg/l: this is close enough to the upper limit of moderate iodine deficiency(Reference Zimmermann31) to suggest that this group is at higher risk for iodine deficiency. While the effects of mild iodine deficiency in pregnant women on maternal and offspring health have not been determined, the risks of moderate–severe iodine deficiency during pregnancy are clear(Reference Markhus, Dahl and Moe32,Reference Charoenratana, Leelapat and Traisrisilp33) .
A moderate–severe iodine deficiency during pregnancy may impair the growth and neurodevelopment of the offspring and increases infant mortality, although this depends on the timing and severity of the hypothyroidism(Reference Zimmermann34,Reference Zimmermann35) . So we suggest that besides improving the USI policy, additional interventions are implemented such as increasing health education and encouraging the intake of supplements or iodine-rich foods in the third trimester(Reference Wang, Zhu and Mo36). In our study, there was a significant difference in UIC between women who took or did not take an iodine-containing supplement, suggesting that supplements should be considered as a source of iodine.
Although rural residents tend to opt for iodised salt, we also found that the median UIC of these individuals was lower than in suburban and rural participants. On the one hand, urban residents in Shanghai eat out more frequently; but on the other hand, with the improvement of social economy and the development of the food industry, global pre-packaged food consumption is increasing(Reference Ng and Dunford37), urban residents may have a higher consumption of pre-packaged food. Iodised salt is used in most pre-packaged food in China. According to a study by the CDC in 2013, the pre-packaged food consumption rate of adult Chinese city residents is 85·3 %. Among these pre-packaged foods, the consumption rates of high-salt convenience foods and baked goods are 52·8 and 31·7 %, respectively(Reference Huang, Zhang and Wang38). Therefore, pre-packaged food is also one of the non-negligible sources of iodised salt. The contribution of iodised salt from pre-packaged foods was not considered in our study, but is worth studying.
To the best of our knowledge, this is the first study that examined the relationship of household cooking salt and eating out on iodine status of pregnant women in environmental iodine-deficient coastal areas. Our research design was reasonable, the sample size was sufficient and the result and conclusion were representative. Our study also had some limitations. We did not test the concentration of salt iodine used in eating out. We could not exclude the use of non-iodised salt or unqualified-iodised salt in centralised meal units, although this was contrary to the national USI policy. On the other hand, we have not investigated the consumption of pre-packaged foods, some of which may use iodised salt.
Based on our findings, iodised salt is important to maintain the iodine intake of pregnant women in Shanghai, but with the increase in eating out intake, the need for using iodised salt as household cooking salt becomes less important. The risk of iodine deficiency in the third trimester was higher than in the first and second trimesters in Shanghai. Additional interventions to encourage the intake of supplements or iodine-rich foods are necessary if a pregnant woman in the third trimester is not eating out and uses non-iodised salt at home.
Acknowledgements
The authors are grateful to the pregnant women who participated in this study and to the healthcare professionals from the CDC of the sixteen districts in Shanghai.
The study was financially supported by the National Nature Science Foundation of China (no. 81602851) and the Excellent Young Talents of Health System in Shanghai (no. 2017YQ043). They had no role in the design, analysis or writing of this article. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
All co-authors contributed significantly to the planning of the analyses and interpretation of the data and provided essential intellectual input. Analysed the data, Z. W. and Z. Z.; investigation, W. J., X. C., Q. S. and Z. S.; project administration, C. G., Z. Z., C. W. and Z. Z.; writing – original draft, Z. W.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S000711452000207X










