Hypercholesterolaemia is a major risk factor for the development of CVD(Reference Neaton, Blackburn and Jacobs1,Reference Tan, Chong and Hamzah2) . Studies have reported that a healthy lifestyle could reduce cardiovascular risk(Reference Köhler, Teupser and Elsässer3). However, limited study has examined the association of meal timing throughout a day with hypercholesterolaemia.
Recent studies have shown that the circadian clock plays a critical role in energy and nutrient metabolism(Reference Jiang and Turek4). It has been reported that feeding and fasting entrain clock genes, which regulate all aspects of metabolism, and meal timing can have serious implications for the development of CVD, type 2 diabetes and obesity(Reference Garaulet and Gómez-Abellán5,Reference Oosterman, Kalsbeek and la Fleur6) . In a current study, the comparison of two isoenergetic weight-loss groups showed greater improvement of metabolic markers in the group haven a bigger breakfast and a smaller dinner(Reference Jakubowicz, Barnea and Wainstein7). Another study demonstrated that early meal timing significantly decreased serum lipids levels(Reference Yoshizaki, Tada and Hida8). Moreover, each 10 % increase in the proportion of total energy intake during the evening was significantly associated with a 3 % increase in C-reactive protein concentrations(Reference Marinac, Sears and Natarajan9). It appears that meal timing can be employed to prevent metabolic pathologies(Reference Asher and Sassone-Corsi10). Nowadays, emerging evidence has suggested that quality and food sources of macronutrients also play crucial roles in human diseases and health(Reference Ludwig, Hu and Tappy11–Reference Reynolds, Mann and Cummings13). Limited studies on the association between the quality and meal timing of macronutrients and foods and health effects have been examined in adults.
In the present study, we aimed to examine the association of energy, macronutrients and food source consumption at breakfast, dinner and their difference (Δratio) with hypercholesterolaemia in adults using data from the National Health and Nutrition Examination Survey (NHANES, 2003–2016).
Methods
Study population
The NHANES is a stratified, multistage study using a nationally representative sample population of the USA(Reference Johnson, Paulose-Ram and Ogden14). Detailed information has been described in detail elsewhere(Reference Shan, Rehm and Rogers15). The present analysis included adults who were aged 20 years or older and completed at least one valid dietary recall during the seven cycles of NHANES from 2003–2004 to 2015–2016. We excluded participants with extreme daily energy intake (<2,092 kcal/d or >14,644 kcal/d for women and <3,347·2/d or >17,572·8 kcal/d for men), pregnant women and participants with missing value on current drinking, current smoking and BMI data. Finally, a total of 27 911 participants were included. The NHANES protocol was approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent.
Dietary assessment
Individuals’ food consumption for two non-consecutive days through 24-h dietary recall interviews were collected. The first 24-h dietary recall was provided in person, and the second 24-h dietary recall was 3–10 d later by telephone. We calculated dietary nutrients and energy consumption using the United States Department of Agriculture’s (USDA) Food and Nutrient Database for Dietary Studies. The mean values for day one and day two of the 24 h dietary recall were used in the present analysis.
Based on the MyPyramid Equivalents Database 2.0 for USDA Survey Foods (MPED 2.0), the dietary intake component of the NHANES was integrated into thirty-seven MyPyramid subgroups. Similar kinds of food were merged into the same group. At last, a total of four main subcategories and seventeen food groups were derived(Reference Rehm, Peñalvo and Afshin16), which were high-quality carbohydrates including whole grains, legumes, whole fruits and non-starchy vegetables; low-quality carbohydrates including refined grains, fruit juice, potato, other starchy vegetables and added sugars; animal protein including red meat, processed meat, poultry, seafood, dairy products and eggs; plant protein including whole grains, refined grains, legumes, nuts and soya. Details are shown in online Supplementary Table 1. Food sources of fat were not examined because they are similar to protein, and existing evidence on fat is mostly focused on types of fatty acids rather than food sources(Reference Sacks, Lichtenstein and Wu17).
Main exposure
The main exposure variable in this study was the ratio of breakfast, the ratio of dinner and Δratio of energy and macronutrients. For example, we calculated the ratio of high-quality carbohydrates at breakfast = high-quality carbohydrates consumption at breakfast/total high-quality carbohydrates; the ratio of high-quality carbohydrates at dinner = high-quality carbohydrates consumption at dinner/total high-quality carbohydrates and Δratio of high-quality carbohydrates = the ratio of high-quality carbohydrates at dinner – the ratio of high-quality carbohydrates at breakfast. ΔRatio of macronutrients in this study included carbohydrates (high-quality carbohydrates and low-quality carbohydrates), fat (SFA and unsaturated fatty acids) and protein (animal protein and plant protein). For participants skipping breakfast, the calculation of the ratio of breakfast was considered as zero intake. Food consumption at breakfast, food consumption at dinner and Δfood were also examined (Δfood = food at dinner – food at breakfast), which means how much more of this food is eaten for dinner than breakfast.
Main outcome
Our primary outcome was hypercholesterolaemia, which was defined as total cholesterol ≥240 mg/dl or current self-reported hypercholesterolaemia. A further detailed description of examination protocol, quality control and safety procedures can refer to the Anthropometry Procedures Manual via the NHANES website.
Assessment of covariates
Potential covariates in this study included age (years), sex (male/female), race/ethnicity (non-Hispanic white/non-Hispanic black/Mexican American/other), education level (< 9th grade/9–11th grade/high school graduate/GED or equivalent/some college or Associate in Arts degree/college graduate or above), annual household income (< $20 000/$20 000–$45 000/$45 000–$75 000/> $100 000), exercise regularly (yes/no), current smoker (yes/no), current drinker (yes/no), prescription medicine use for lower blood sugar, prescription medicine use for hypertension, prescription medicine use for cholesterol; total intake of energy (kcal/d), fat (g/d), protein (g/d), SFA (g/d), dietary fibre (g/d), dietary cholesterol (mg/d), high-quality carbohydrates and animal protein, dietary supplements use (yes/no), breakfast skipping (yes/no) and diet quality calculated by the alternative healthy eating index(Reference Wang, Leung and Li18).
Statistical analyses
All analyses incorporated the dietary sample weights, stratification and clustering of the complex sampling design to ensure nationally representative estimates according to NHANES analytic guidelines(Reference Johnson, Paulose-Ram and Ogden14). The absolute consumption of macronutrients and foods per day was adjusted for total energy by the residual method to correct for measurement error in dietary estimates(Reference Willett, Howe and Kushi19).
The differences in the Δratio, the ratio of breakfast and the ratio of dinner of total energy, macronutrients and subcategories of macronutrients were categorised into quartiles. The differences in Δfoods, foods at breakfast and foods at dinner were considered continuous variables. Demographic characteristics, dietary nutrient consumption, lifestyle and anthropometric measurements were shown as means and standard error for continuous variables and percentage for categorical variables. General linear models were performed to test the differences of Δratio of energy by quartile adjusting for age and Chi-squared test for continuous and categorical variables, respectively.
Multivariable logistic regression models were performed to explore the association between the Δratio, the ratio of breakfast and the ratio of dinner of energy, macronutrients and subcategories of macronutrients with hypercholesterolaemia. The association of Δfoods, foods at breakfast, foods at dinner and risk of prevalent hypercholesterolaemia was also examined by logistic regression models. OR and 95 % CI were provided. Models were adjusted for age, sex, ethnicity, income, education, exercise, smoke, alcohol intake, BMI, prescription medication used for lower blood sugar, prescription medication used for hypertension and prescription medication used for cholesterol, total intake of energy, fat, protein, SFA, high-quality carbohydrates, animal protein, dietary fibre, dietary cholesterol, alternative healthy eating index, supplement use and breakfast skipping. To test for linear trends, we modelled categorical variables as continuous by assigning the median value to each quintile. For each Δfood, food at breakfast and food at dinner, models were additionally adjusted for the total daily intake of the food consumption.
We further examined the substitution effects of replacing 1 cup/ounce per tsp equivalent of food consumption at dinner with breakfast. For each substitution of dinner with breakfast, the food consumption at breakfast and dinner as well as other variables was all included in the same multivariable models as continuous(Reference Kulldorff, Sinha and Chow20,Reference Hu, Stampfer and Rimm21) . It statistically predicted the substitution effects on the risk of prevalent hypercholesterolaemia. This substitution is being interpreted as the risk of prevalent hypercholesterolaemia associated with decreased food consumption at dinner and simultaneously increased food consumption at breakfast. Models were adjusted for age, sex, ethnicity, income, education, exercise, smoke, alcohol intake, BMI, prescription medication used for lower blood sugar, prescription medication used for hypertension, prescription medication used for cholesterol, total intake of energy, fat, protein, SFA, high-quality carbohydrates, animal protein, dietary fibre, dietary cholesterol, alternative healthy eating index, supplement use and breakfast skipping. For each food, models were additionally adjusted for the total daily intake of this kind of food.
Four sensitivity analyses were performed: (1) multiple logistic regression models and substitution effects were performed in participants without breakfast skipping, which was a traditional health-related dietary factor; (2) multiple logistic regression models were performed to examine the association between percentage energy from macronutrients at breakfast, dinner and Δ(dinner – breakfast) (subcategories of macronutrients were measured in energy-adjusted form by units per 1000 kcal per day according to the previous study(Reference Zhong, Van Horn and Greenland22) and the risk of prevalent hypercholesterolaemia; (3) multiple logistic regression models were performed to examine the association between Δratio of energy and subcategories of macronutrients with hypercholesterolaemia in participants who provided LDL-cholesterol and HDL-cholesterol level, further adjustment with non-HDL-cholesterol = total cholesterol – HDL-cholesterol (hypercholesterolaemia was defined as total cholesterol ≥240 mg/dl or current self-reported hypercholesterolaemia or LDL-cholesterol > 160 mg/dl); and (4) multiple logistic regression models and substitution effects were performed further adjusted with fasting time.
A two-sided P value < 0·05 was considered statistically significant. All analyses were performed by R 3.6.1(www.r-project.org/).
Results
Characteristics of participants
Online Supplementary Table 2 illustrates the characteristics among 27 911 participants aged over 20 years of NHANES 2003–2016 in this study. Among the participants, 11 697 hypercholesterolaemia was documented. Compared with participants in the lowest quintile, those in the highest quintile were more likely to be younger, men, non-Hispanic white, current smokers, current drinkers, with higher waist circumstance and higher energy consumption at dinner, but with lower energy consumption at breakfast, lower dietary fibre and lower dietary cholesterol consumption. No significant difference in BMI, exercise regularly, the prescription medicine used for hypertension and prescription medicine used for cholesterol across these quartiles was observed.
Association of ratio at breakfast, ratio at dinner and Δratio of energy and macronutrients with the risk of prevalent hypercholesterolaemia
Association of ratio at breakfast, ratio at dinner and Δratio of energy and macronutrients with the risk of prevalent hypercholesterolaemia is shown in Table 1. As indicated by OR and 95 % CI, the highest quintile of energy consumption at breakfast was significantly associated with decreased risk of prevalent hypercholesterolaemia (OR 0·75, 95 % CI (0·65, 0·87)). Fat and protein consumption at breakfast was related to reduced hypercholesterolaemia risk (ORfat 0·79, 95 % CI (0·67, 0·93)) and (ORprotein 0·76, 95 % CI (0·64, 0·89)). No significant association was observed for the ratio at dinner. Further, participants in the highest quintile of Δratio in terms of energy and carbohydrates had a higher risk of prevalent hypercholesterolaemia (ORenergy 1·16, 95 % CI (1·01, 1·33); P for trend = 0·039) and (ORcarbohydrates 1·15, 95 % CI (1·01, 1·31); P for trend = 0·022).
Table 1. Association between the ratio of energy and macronutrient consumption at breakfast, dinner and Δratio and the OR of being hypercholesterolaemia† (Odds ratios and 95 % confidence intervals)

AHEI, alternative healthy eating index; Q, quintile.
* P < 0·05.
† Models were adjusted for age, sex, ethnicity, income, education, exercise, smoke, alcohol intake, BMI, prescription medication used for lower blood sugar, prescription medication used for hypertension and prescription medication used for cholesterol, total intake of energy, fat, protein, SFA, high-quality carbohydrate, animal protein, dietary fibre, dietary cholesterol, AHEI, supplement use and breakfast skipping.
Association of ratio at breakfast, ratio at dinner and Δratio of subcategories of macronutrients with the risk of prevalent hypercholesterolaemia
The association of ratio at breakfast, ratio at dinner and Δratio of subcategories of macronutrients with the risk of prevalent hypercholesterolaemia is shown in Table 2. After adjustment for a series of potential covariates, low-quality carbohydrates, animal protein and plant protein consumption at breakfast were related to reducing the risk of prevalent hypercholesterolaemia risk (ORlow-quality carbohydrates 0·86, 95 % CI (0·76, 0·97)), (ORanimal protein 0·82, 95 % CI (0·72, 0·94)) and (ORplant protein 0·80, 95 % CI (0·70, 0·91)). No significant association was observed for the ratio at dinner, whereas Δratio of low-quality carbohydrates and plant protein were more likely to be hypercholesterolaemia (ORlow-quality carbohydrates 1·19, 95 % CI (1·05, 1·35); P for trend = 0·004) and (ORplant protein 1·13, 95 % CI (1·01, 1·28); P for trend = 0·043).
Table 2. Association between the ratio of subcategories of macronutrient consumption at breakfast, dinner and Δratio and the OR of being hypercholesterolaemia†
(Odds ratios and 95 % confidence intervals)
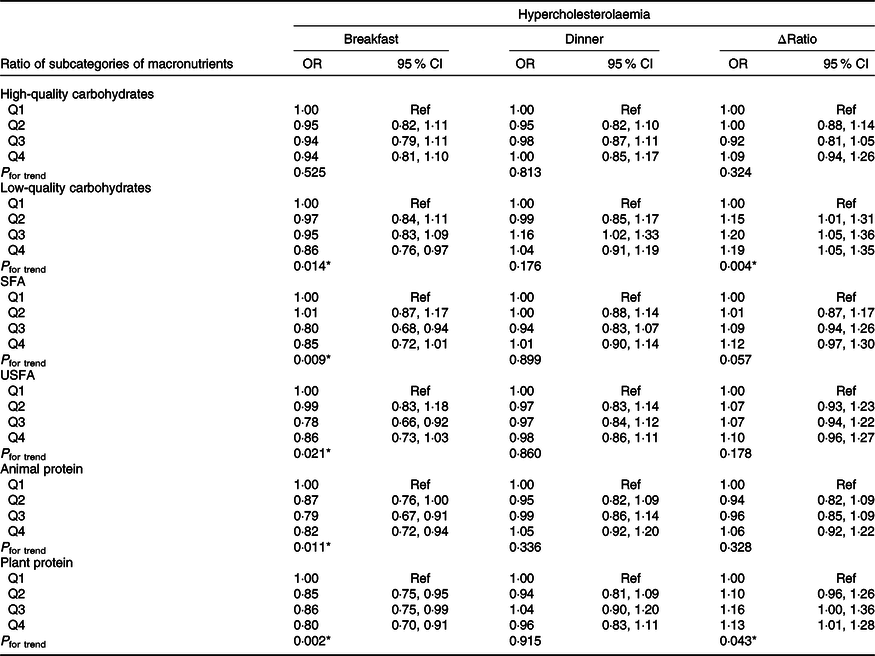
USFA, unsaturated fatty acid; AHEI, alternative healthy eating index; Q, quintile.
* P < 0·05.
† Models were adjusted for age, sex, ethnicity, income, education, exercise, smoke, alcohol intake, BMI, prescription medication used for lower blood sugar, prescription medication used for hypertension and prescription medication used for cholesterol, total intake of energy, fat, protein, SFA, high-quality carbohydrate, animal protein, dietary fibre, dietary cholesterol, AHEI, supplement use and breakfast skipping.
Association of foods at breakfast, foods at dinner and Δfoods with the risk of prevalent hypercholesterolaemia
Fig. 1 shows the association of foods at breakfast, foods at dinner and Δfoods with the risk of prevalent hypercholesterolaemia. For low-quality carbohydrate foods, added sugar consumption at breakfast was marginally associated with decreased risk of prevalent hypercholesterolaemia (OR 0·98; 95 % CI (0·97, 0·99)). No significant association was observed for dinner. However, Δadded sugar was associated with a higher risk of prevalent hypercholesterolaemia (OR 1·01; 95 % CI (1·00, 1·02)). Similarly, for plant protein, nuts consumption at breakfast was associated with a decreased risk of prevalent hypercholesterolaemia (OR 0·82; 95 % CI (0·73, 0·92)). No significant association was observed for dinner. And Δnuts consumption was associated with a higher risk of prevalent hypercholesterolaemia (OR 1·08; 95 % CI (1·01, 1·16)).

Fig. 1. The association of Δfoods, foods at breakfast and foods at dinner with hypercholesterolaemia.
Substitution effects of replacing one cup/ounce per tsp foods consumption at dinner with breakfast with the risk of prevalent hypercholesterolaemia
Table 3 shows the substitution effects with a decrease of one cup/ounce per tsp foods consumption at dinner and a simultaneous increase of one cup/ounce per tsp foods consumption at breakfast with the risk of prevalent hypercholesterolaemia. Overall, we found a tsp equivalent decrease in added sugar consumption at dinner with a tsp equivalent increase at breakfast was associated with a lower risk of prevalent hypercholesterolaemia (OR 0·98; 95 % CI (0·97, 0·99)). An ounce equivalent decrease in processed meat consumption at dinner with an ounce equivalent increase at breakfast could reduce the risk of prevalent hypercholesterolaemia by 4 % (OR 0·96; 95 % CI (0·93, 1·00)). Also, an ounce equivalent decrease in nuts consumption at dinner with an ounce equivalent increase at breakfast could reduce the risk of prevalent hypercholesterolaemia by 17 % (OR 0·83; 95 % CI (0·74, 0·94)).
Table 3. Substitution effects of replacing one cup/ounce per tsp foods consumption at dinner with breakfast with the risk of prevalent hypercholesterolaemia†
(Odds ratios and 95 % confidence intervals)

AHEI, alternative healthy eating index.
* P < 0·05.
† Models were adjusted for age, sex, ethnicity, income, education, exercise, smoke, alcohol intake, BMI, prescription medication used for lower blood sugar, prescription medication used for hypertension, prescription medication used for cholesterol, total intake of energy, fat, protein, SFA, high-quality carbohydrates, animal protein, dietary fibre, dietary cholesterol, AHEI, supplement use and breakfast skipping. For each food, models were additionally adjusted for the total daily intake of this kind of food.
Sensitivity analyses
After excluding participants with breakfast skipping, the above associations with the risk of prevalent hypercholesterolaemia were consistent with those from the primary analyses of complete participants (online Supplementary Tables 3–5; online Supplementary Fig. 1). Similar associations were also observed between percentage energy from macronutrients and hypercholesterolaemia, as well as subcategories of macronutrients were measured in energy-adjusted form (online Supplementary Tables 6–7). The associations remained robust among participants who provided LDL-cholesterol and HDL-cholesterol levels further adjustment with non-HDL-cholesterol, in which hypercholesterolaemia was defined as total cholesterol ≥240 mg/dl or current self-reported hypercholesterolaemia or LDL-cholesterol > 160 mg/dl (online Supplementary Table 8). The associations were consistent with the primary outcomes when further adjusted with fasting time (online Supplementary Tables 9–11, online Supplementary Fig. 2).
Discussion
In this nationally representative sample of US adults, this study demonstrated that excessive energy consumption at dinner than breakfast throughout the day was associated with an increased risk of prevalent hypercholesterolaemia mainly due to low-quality carbohydrates and plant protein. Overconsumption of added sugars and nuts at dinner than breakfast was associated with hypercholesterolaemia. Furthermore, replacing added sugar, nuts and processed meat at dinner with breakfast could reduce the prevalent hypercholesterolaemia risk. This study emphasised the importance of meal timing in the prevention of hypercholesterolaemia.
To the best of our knowledge, this was the first study that examined the association of energy, macronutrients and food sources with the risk of prevalent hypercholesterolaemia by considering the quality and meal timing simultaneously based on national-scale representative data. We observed that excess energy consumption at dinner was significantly associated with an increased risk of prevalent hypercholesterolaemia, mainly due to overconsumption of low-quality carbohydrates and plant protein at dinner. In several cross-sectional studies, late-night eating has been associated with a higher risk of poor cardiometabolic health(Reference St-Onge, Ard and Baskin23). Late-night eating was associated with an OR for obesity (OR 1·62; 95 % CI (1·10, 2·39)) compared with no late-night eating among Swedish men and women(Reference Berg, Lappas and Wolk24). Additionally, participants who ate at night and skipped breakfast had an OR for the metabolic syndrome (OR 1·17; 95 % CI (1·08, 1·28)) among the Japanese population(Reference Kutsuma, Nakajima and Suwa25). Moreover, animal studies have observed that higher energy consumption at breakfast related to the circadian phasing of peripheral clocks in the liver with improved blood lipids(Reference Ruddick-Collins, Johnston and Morgan26,Reference Kuroda, Tahara and Saito27) , while higher energy consumption at dinner was associated with lipid metabolism and adipose tissue accumulation(Reference Wu, Sun and ZhuGe28,Reference Wang, Xue and Yang29) . Also, increasing energy proportion at breakfast and reducing them at dinner could restore clock gene expression, leading to decreased blood lipids and body weight(Reference Wu, Sun and ZhuGe28,Reference Fuse, Hirao and Kuroda30,Reference Sherman, Genzer and Cohen31) . All in all, accumulated evidence has suggested the benefits of bigger energy consumption at breakfast.
Moreover, this study also observed the harmful effects of overconsumption of added sugars and nuts at dinner. As we know, added sugars have been related to CVD risk(Reference Yang, Zhang and Gregg32). Research has reported that excessive consumption of added sugar was significantly associated with increased de novo lipogenesis in the liver, hepatic TAG synthesis and increased TAG levels(Reference Johnson, Appel and Brands33,Reference Fried and Rao34) . One proposed pathway of the association between overconsumption of added sugars and increased CVD risk is mainly due to inflammation markers, which are key factors in the pathogenesis of CVD(Reference Malik, Popkin and Bray35–Reference Pearson, Mensah and Alexander37). Furthermore, we observed that higher nuts consumption at dinner than breakfast was associated with the prevalent hypercholesterolaemia risk. There has been reported that nuts consumption at breakfast was related to less abdominal obesity(Reference Chatelan, Castetbon and Pasquier38). This association was consistent with our analyses of nuts consumption at breakfast with hypercholesterolaemia. This suggested that eating nuts at breakfast is beneficial instead of dinner.
Substitution effects with one cup/ounce per tsp decrease of added sugar, processed meat and nuts consumption at dinner and a simultaneous increase at breakfast reduced the risk of prevalent hypercholesterolaemia. This observation additionally suggested the critical role of meal timing on health effects throughout the day. Studies have reported that meal timing could influence inflammation, insulin sensitivity and lipid profiles(Reference Mattson, Longo and Harvie39–Reference Patterson and Sears41). Also, the above associations were consistent with our primary analyses when further consideration of breakfast skipping and dietary quality(Reference St-Onge, Ard and Baskin23,Reference Haslam and James42) , which were traditional health-related dietary factors. Our study suggests that the meal timing needs to be taken into consideration for dietary recommendations for hypercholesterolaemia prevention.
There were several strengths in this present analysis. First, this was the first study that examined the association of energy, macronutrients and food sources meal timing throughout the day with hypercholesterolaemia based on national-scale representative data from the well-designed study (NHANES). Second, the association remained robust when additional consideration of a series of dietary confounders, such as breakfast skipping and dietary quality. Of course, there were several limitations. First, measurement error was unavoidable for diet and other information. This may result in a misestimation of an association. Second, a variety of confounders were included, but residual confounding was still likely existed. Third, causality inference is limited due to the cross-sectional study design of this study. At last, self-reported dietary 24-h recall data are subject to measurement error due to large day-to-day variations in food intake.
Conclusions
This study indicated that among US adults, overconsumption of energy, macronutrients including low-quality carbohydrates and plant protein at dinner than breakfast was significantly associated with a higher risk of prevalent hypercholesterolaemia. Replacing added sugar, nuts and processed meat at dinner with breakfast could reduce the risk of prevalent hypercholesterolaemia. This study emphasised the importance of meal timing in the prevention of hypercholesterolaemia.
Acknowledgements
The authors thank the participants and staff of the National Health and Nutrition Examination Survey 2003–2016 for their valuable contributions.
This study was supported by funds from the National Natural Science Foundation of China (82073534 to Changhao Sun).
All authors made a significant contribution to this article. C. H. S. and W. Y. H. planned the work. X. N. L. and T. S. H. carried out the statistical analysis. Y. Y.C., X. Y. S., J. X. X. and Y. F. M. wrote and reported the work. All authors critically assessed and reviewed the paper and approved the version to be published. C. H. S. and W. Y. H. are responsible for the overall content as guarantors.
The authors declare that they have no competing interests with this manuscript.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114522003257









