Progressive multifocal leukoencephalopathy (PML) is a rare opportunistic infection of the central nervous system caused by John Cunningham virus (JCV), a polyomavirus (Reference Koralnik1). It primarily occurs in patients with immune deficiency such as malignancies, HIV infection, or immunosuppressive therapy (Reference Jelcic, Jelcic, Faigle, Sospedra and Martin2). Reports of PML in patients without clear immunodeficiency are extremely rare (Reference Gheuens, Pierone and Peeters3). Here, we describe the case of an 87-year-old man with chronic renal failure on hemodialysis and no clear immune suppression who presented with progressive neurological decline, and post mortem studies led to the diagnosis of PML.
An 87-year-old male, with end-stage renal disease on hemodialysis and multiple vascular risk factors, presented with 2-week history of right arm weakness and dysarthria. Initial computed tomography (CT) scan of the head demonstrated no acute abnormality. Magnetic resonance imaging (MRI) of the brain (diffusion-weighted imaging (DWI) only) demonstrated a right cerebellar lesion which was interpreted as a subacute infarct (Figure 1A-C). Clopidogrel was added to his home antiplatelet (Aspirin), stroke workup was initiated, and he was transferred to stroke rehabilitation.
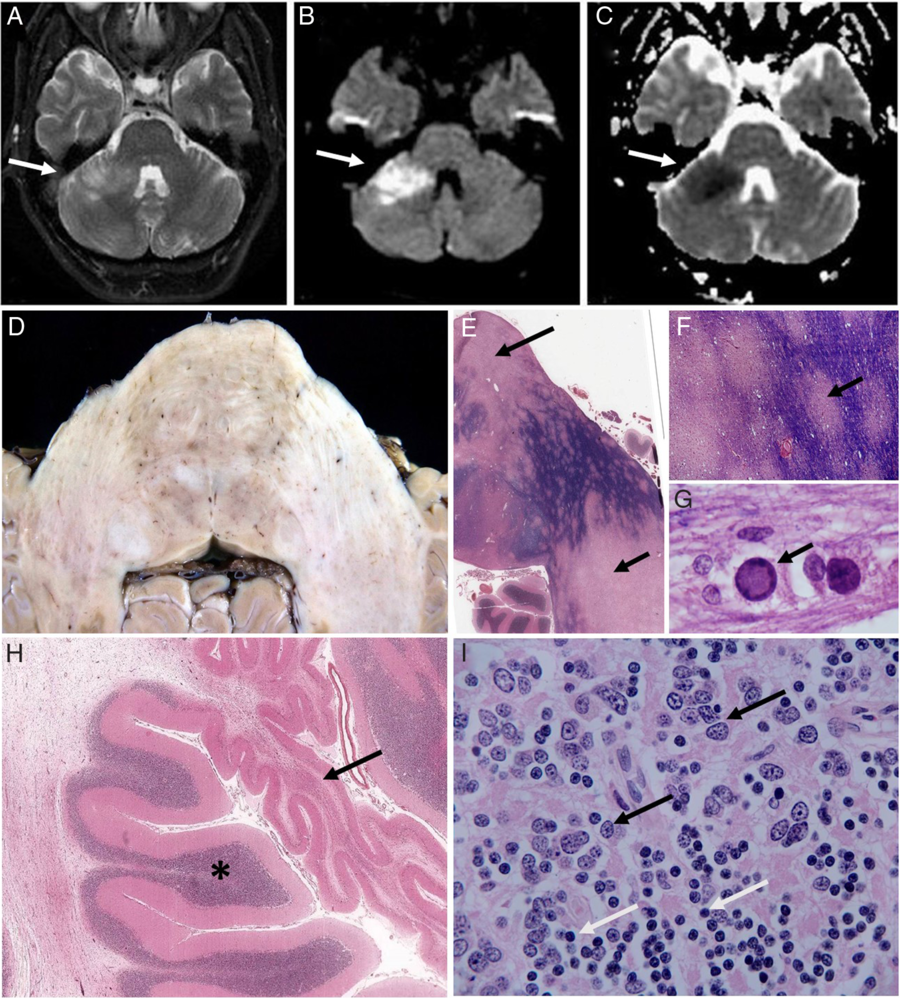
Figure 1: Axial MRI of the brain (A–C), with the right cerebellar lesion (arrows) appearing bright on diffusion-weighted imaging (DWI) sequences (A,B) and dark on apparent diffusion coefficient (ADC) sequence (C). Axial sections (D–I) showing subtle mottling of the base of the pons and middle cerebellar peduncles (D), with large pale areas of established demyelination (E, arrows) and multiple small pale foci of active demyelination (E and enlarged area F, arrow) with Solochrome Cyanine R [SCR] staining (intact myelin blue). Enlarged oligodendrocyte nuclei (arrow) containing amphophilic material (G) in area of active demyelination (SCR stain). Cerebellum (H), with atrophied area due to complete loss of internal granule cells (H, arrow; adjacent normal appearing cortex, asterisk) and a group of enlarged JCV-infected granule cells (I, representative infected cells, black arrows, compared to normal appearing cells, white arrows).
Four weeks later, he received a percutaneous gastric tube due to progressive dysphagia. His Holter monitor revealed new atrial fibrillation.
In less than a week, he was readmitted with progressive right-sided weakness, worsening dysphagia, and aspiration pneumonia. Despite treatment with moxifloxacin, he continued to have gradual neurological decline, and repeat MRI revealed that the previous area of high signal intensity in the right brachium pontis and cerebellum had progressed. New patchy areas of signal changes were seen in the left and central pons and cerebellum.
MRI of the brain with gadolinium was subsequently performed. The lesions were reported as being unusual in appearance for ischemic changes, with a differential diagnosis including osmotic demyelination and inflammatory or infectious etiologies. He was started on moxifloxacin 400 mg IV daily (the patient had documented allergies to penicillin and cephalexin).
Lumbar puncture showed WBC: 1 × 106/L, RBC: 9 × 106/L, glucose: 7 mmol/L (H), protein: 0.61 g/L (H) (reference range: 0.15–0.45 g/L); cerebrospinal fluid (CSF) gram stain, cytology, and polymerase chain reaction (PCR) for CMV, EBV, HSV1/2 and VZV, were negative.
Antimicrobial therapy was discontinued. Repeat CT scan of the brain showed expansion of the abnormal signal. Empiric high-dose IV steroid therapy was initiated, but he had continued clinical deterioration. A brain biopsy was discussed, but the patient requested the transition to comfort care only. He died 77 days following his initial presentation, and a consented autopsy was performed.
Macroscopic examination of the brain showed subtle mottling of the base of the pons, middle cerebellar peduncles, and paramedian cerebellar white matter bilaterally (Figure 1D). Microscopically, there was extensive established demyelination in the pons, right middle cerebellar peduncle, and paramedian cerebellum bilaterally (Figure 1E). There were multiple small foci of active ongoing demyelination in the left middle cerebellar peduncle (Figure 1F), and in these areas, there were numerous enlarged oligodendrocyte nuclei containing amphophilic material (Figure 1G), characteristic of JCV infection. Frequent hypertrophic astrocytes with large pleomorphic nuclei (another characteristic feature of PML) and numerous debris-filled macrophages were present in areas of established demyelination. There was JCV-related cerebellar granule cell neuronopathy, which focally was severe with complete loss of internal granule cells (Figure 1H), while elsewhere in the cerebellar cortex there were multifocal groups of enlarged JCV-infected granule cells (Figure 1I). There was multifocal demyelination in the rostral medulla oblongata, but only small microscopic foci in the caudal medulla and midbrain, and no evidence of PML in supratentorial structures. Stored CSF was sent for JC virus PCR, which was positive.
In our review of the literature, we identified five cases of PML-reported patients with chronic renal failure, three on hemodialysis, without overt immune suppression (Table 1).
Table 1: Reported cases of PML in patients with chronic renal failure

HD: hemodialysis; NA: not applicable; NR: not reported.
There are reports that patients with chronic renal disease, in the absence of kidney transplantation or immune suppression, are at higher risk of developing malignant tumors (Reference Cengiz4), suggesting that chronic renal failure may represent a state of subtle immune suppression. A study of 38 patients with end-stage renal disease demonstrated altered balance of T cell-associated immunity compared to controls, particularly with lowered naïve T cell percentage and increased percentage of Th17-producing effector memory cells and central memory T cells (Reference Chung, Kim and Sun5). The exact mechanism of JCV reactivation in chronic renal failure has not been established.
PML commonly affects white matter of the cerebral hemispheres, and isolated posterior fossa manifestations are uncommon (Reference Berger, Aksamit and Clifford6), although cases have been described (Reference Aotsuka, Uzawa and Nishimura7). JCV classically causes lytic infection of astrocytes and oligodendrocytes. Similar to our case, JCV may also infect cerebellar granule cell neurons (granule cell neuronopathy) leading to selective neuronal loss and gliosis (Reference Wüthrich, Cheng and Joseph8).
Our case highlights that although PML is a rare disease, it should be considered in patients with chronic renal failure, especially those on hemodialysis, who present with progressive unexplained neurological symptoms.
Acknowledgement
The authors thank the patient’s next of kin for consenting to the publication of this case report.
Conflict of Interest
The authors declare no competing interests relevant to this work.
Statement of Authorship
All authors have seen and approved the content of the submission, and all have contributed significantly to the work. B.D. and M.B. were involved in the clinical care of the patient, prepared the written case report, and performed the initial literature review. J.R. performed neuropathological analysis of tissue, performed additional literature review, prepared histologic images, and reviewed and edited the manuscript.






