Introduction
As the world’s population is aging, so the prevalence of multiple chronic conditions (MCC) in older adults is also increasing on a global scale (Hwang, Weller, Ireys, & Anderson, Reference Hwang, Weller, Ireys and Anderson2001; Schellevis, Reference Schellevis2013). MCC is commonly defined as having two or more chronic conditions that persist for at least a year (Boyd & Fortin, Reference Boyd and Fortin2010; Uijen & van de Lisdonk, Reference Uijen and van de Lisdonk2008). For example, in the United States the prevalence of MCC in adults older than 65 years is more than 80 per cent (Buttorff, Ruder, & Bauman, Reference Buttorff, Ruder and Bauman2017). Older adults with MCC manage conditions such as type 2 diabetes, heart disease, and arthritis, which are associated with poorer health status, higher odds of adverse treatment-related events, and increased health care utilization and associated costs, compared with older adults with single conditions (Bähler, Huber, Brüngger, & Reich, Reference Bähler, Huber, Brüngger and Reich2015; Canadian Institute for Health Information, 2011; Lehnert et al., Reference Lehnert, Heider, Leicht, Heinrich, Corrieri and Luppa2011; Marengoni et al., Reference Marengoni, Angleman, Melis, Mangialasche, Karp and Garmen2011; Skinner, Coffey, Jones, Heslin, & Moy, Reference Skinner, Coffey, Jones, Heslin and Moy2016; Tooth, Hockey, Byles, & Dobson, Reference Tooth, Hockey, Byles and Dobson2008; Wolff, Starfield, & Anderson, Reference Wolff, Starfield and Anderson2002). Studies have shown a link between physical inactivity and the development of chronic diseases. The evidence suggests that higher physical activity (PA) may prevent, delay, or even reverse the progression of these diseases and improve the health of community-dwelling older adults with MCC, hereafter referred to as older adults (Clegg, Barber, Young, Iliffe, & Forster, Reference Clegg, Barber, Young, Iliffe and Forster2014; Miller, Rejeski, Reboussin, Ten Have, & Ettinger, Reference Miller, Rejeski, Reboussin, Ten Have and Ettinger2000; Public Health Agency of Canada, 2013; Tessier et al., Reference Tessier, Menard, Fulop, Ardilouze, Roy and Dubuc2000; World Health Organization, 2005).
Enabling older adults to remain in the community is vital to their health, and PA is a critical factor in supporting this goal. Residing at home optimizes older adults’ health, autonomy, control, and sense of well-being as well as facilitating their social connectedness (Wiles, Leibing, Guberman, Reeve, & Allen, Reference Wiles, Leibing, Guberman, Reeve and Allen2012). A range of PA levels has been shown to increase older adults’ mental and physical functioning and it can be instrumental in helping them maintain their cardiovascular, metabolic, and cognitive health as well as their independence (Bouaziz et al., Reference Bouaziz, Vogel, Schmitt, Kaltenbach, Geny and Lang2017; McPhee et al., Reference McPhee, French, Jackson, Nazroo, Pendleton and Degens2016). Furthermore, PA can help reduce or prevent age-related risks such as falls, and limited PA has been associated with care dependency in older adults (Hopewell et al., Reference Hopewell, Adedire, Copsey, Boniface, Sherrington and Clemson2018; Schnitzer et al., Reference Schnitzer, Blüher, Teti, Schaeffner, Ebert and Martus2019). Research suggests that PA levels are reduced in adults with chronic conditions when compared with those of their healthy counterparts (Barker et al., Reference Barker, Smith Byrne, Doherty, Foster, Rahimi and Ramakrishnan2019). Therefore, supporting older adults, including those with MCC, to best optimize their health through PA and other health management strategies, is an important goal of health promotion and health management initiatives. Validated PA measures such as self-report PA tools, which can be completed by older adults in their home environment, are well-placed to assess and monitor PA over time. Determining which self-report PA tool is best suited for use in older adults is important to ensure that clinicians are able to accurately assess PA changes in a consistent and reliable manner.
Although many older adults with MCC face challenges related to engaging in PA, PA continues to be a positive influential factor in maintaining or enhancing their physical and psychological health (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). Arthritis diabetes, and heart disease are the most commonly occurring chronic conditions in older adults, and these conditions can be prevented, improved, or modified by PA (Canadian Institute for Health Information, 2011; Tessier et al., Reference Tessier, Menard, Fulop, Ardilouze, Roy and Dubuc2000; Thompson et al., Reference Thompson, Buchner, Pina, Balady, Williams and Marcus2003). Nevertheless, studies have shown that although many older adults with MCC have the ability to meet recommended activity levels for their age, they consistently fail to reach these targets (Ashe, Eng, Miller, & Soon, Reference Ashe, Eng, Miller and Soon2007).
Therefore, increasing older adults’ PA levels in support of disease management and health promotion is important for managing chronic conditions in this population (Canadian Institute for Health Information, 2011). Unfortunately, evaluation of PA as part of chronic disease management programs is often hindered by limited availability of reliable data for assessing PA and older adults’ PA progress over time (Sun, Norman, & While, Reference Sun, Norman and While2013).
Background
Direct and objective PA instruments assess PA levels using biological or behavioural markers. The gold standard for directly determining PA is through assessment of total energy expenditure with doubly labeled water (DLW). The use of DLW and other objective PA instruments such as motion sensors, heart rate monitors, and measurement of maximum oxygen capacity (VO2 max) may be limited by investigator, equipment, participant availability, time constraints, and subject PA capability (Shephard, Reference Shephard2003; Washburn, Reference Washburn2000). Furthermore, the substantial cost and logistics such as delivery and pick-up of PA measurement equipment can restrict their widespread use (Washburn, Smith, Jette, & Janney, Reference Washburn, Smith, Jette and Janney1993). Although other objective measures such as accelerometers and pedometers also provide objective measurements of PA at lower cost, they may not capture PA that involves body parts beyond the lower extremities. In addition, accelerometers also require the availability of advanced computer software to access their data, and pedometers cannot capture changes in behaviour patterns or intensity (Castillo-Retamal & Hinckson, Reference Castillo-Retamal and Hinckson2011). Validated and reliable self-report PA tools can provide a low-cost, functional means of capturing varying levels of activity over time, making them appropriate for use in community-dwelling older adults with MCC.
Self-report PA tools use recall to provide subjective measures of PA based on the use of questionnaires, diaries, interviews, or other means, reporting on levels and patterns of PA. Self-report PA tools allow for measurement of population-wide activity levels, which can be used to monitor changes in behaviour over time and as a means of testing whether an intervention has resulted in a change in behaviour (Castillo-Retamal & Hinckson, Reference Castillo-Retamal and Hinckson2011; Prince et al., Reference Prince, Adamo, Hamel, Hardt, Connor Gorber and Tremblay2008). Such tools have widespread application in tracking population rates of morbidity and mortality by public health personnel as well as in monitoring progress in the context of an individualized care plan at the level of primary care (Prince et al., Reference Prince, Adamo, Hamel, Hardt, Connor Gorber and Tremblay2008). Furthermore they can be used to classify groups of people according to variables such as activity level or duration of exercise, allowing for comparisons among different groups of people and exploration of potential associations between factors such as activity level and insulin sensitivity or triglyceride levels (Lakka et al., Reference Lakka, Lakka, Rankinen, Leon, Rao and Skinner2005; Martins et al., Reference Martins, Aguiar, Nadeau, Scianni, Teixeira-Salmela and Coelho de Morais Faria2017). These indirect measures of PA have increased in popularity because of their affordability, convenience, low participant burden, and ability to capture detailed information about PA, including activities of daily living and leisure-related exercise (Dishman, Washburn, & Shoeller, Reference Dishman, Washburn and Schoeller2001).
It is increasingly recognized that chronic disease management strongly depends on self-management approaches, of which PA is an essential component (Bodenheimer, Lorig, Holman, & Grumbach, Reference Bodenheimer, Lorig, Holman and Grumbach2002). This means that older adults themselves should also have access to simple, effective, evidence-based PA tools. These tools should be sensitive to a variety of PA activities, including low-intensity PA, which these older adults can use to track their progress, and which are sustainable over time. Pedometers are only able to measure bipedal mobility and cannot capture change in intensity of PA. Accelerometers may also represent challenges when measuring PA in people with mobility issues, because they cannot be used in water and cannot measure activity that does not include acceleration (Kowalski, Rhodes, Naylor, Tuokko, & MacDonald, Reference Kowalski, Rhodes, Naylor, Tuokko and MacDonald2012). In contrast, self-report PA tools are able to assess different types of PA such as swimming or cycling, which are both age-friendly forms of PA. Furthermore, these questionnaires can detect different levels of intensity, and are widely used in research involving population level studies and epidemiological assessments, although there is research suggesting that they may overestimate PA and underestimate inactive time (Schrack et al., Reference Schrack, Cooper, Koster, Shiroma, Murabito and Rejeski2016; Steene-Johannessen, et al., Reference Steene-Johannessen, Anderssen, van der Ploeg, Hendriksen, Donnelly and Brage2016). Self-report PA tools may be well placed to assess PA in older adults by providing them, as well as their health providers, with an affordable, easily administered alternative that is sensitive to the type of PA engaged in by older adults rather than with costly and complex objective measures such as doubly labelled water or accelerometers. Being able to track PA over time is important in the context of older adults with MCC for various reasons: (1) clinicians need valid and reliable measures of clients’ engagement in health management behaviours, (2) monitoring health behaviours can assist older adults in meeting health management goals, and (3) researchers require access to validated, reliable tools in order to better understand the effects of PA; for example, by assessing relative changes in PA at the population level in older adults with MCC (Marengoni et al., Reference Marengoni, Angleman, Melis, Mangialasche, Karp and Garmen2011; Merom et al., Reference Merom, Delbaere, Cumming, Voukelatos, Rissel and Van Der Ploeg2014; Portegijs, Sipilä, Viljanen, Rantakokko, & Rantanen, Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017; Vancampfort, Stubbs, & Koyanagi, Reference Vancampfort, Stubbs and Koyanagi2017). However, application of self-report PA tools for all these purposes requires ensuring that these tools are amenable for use and sensitive to the activities engaged in by older adults who may be managing several physical and cognitive challenges (Merom et al., Reference Merom, Delbaere, Cumming, Voukelatos, Rissel and Van Der Ploeg2014).
Many self-report PA tools are available for use in the adult population (persons ≥ 18 years of age) and several tools have been developed specifically for the older adult population (≥ 65 years of age), such as the Community Healthy Activities Model Program for Seniors (CHAMPS), the Physical Activity Scale for the Elderly (PASE), the Single Self-Report Physical Activity Question (SR-PA), and the Yale Physical Activity Survey (YPAS) (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017; Rosario, Vazques, Cruz, & Ortiz, Reference Rosario, Vazquez, Cruz and Ortiz2008; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). Research has also shown that PA self-report tools have demonstrated preliminary success as an adjunct to counselling in modifying health behaviours in primary care settings (Petrella & Lattanzio, Reference Petrella and Lattanzio2002; Spink & Wilson, Reference Spink and Wilson2010). Although to date most PA self-report tools are implemented in the context of research studies, especially larger scale epidemiological research, there is increasing emphasis being placed on the need for clinicians to prescribe and document regular PA as an integral part of primary care (Rippe, Reference Rippe2018). However, little is known about the psychometric properties and feasibility of using self-report PA tools in the older adult population (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). Moreover, self-report PA tools that have been validated in healthy older adults may be ineffective in capturing the amount of and capacity for PA in older adults with MCC. A review by Forsen et al. (Reference Forsen, Loland, Vuillemin, Chinapaw, Van Poppel and Mokkink2010) reported that although associations were found between self-report PA tool results and health variables, limited knowledge was available on the reliability and validity of these tools in older adults with MCC, a population that engages in limited PA (Hung, Ross, Boockvar, & Siu, Reference Hung, Ross, Boockvar and Siu2011). The feasibility of using self-report PA tools for older adults, a critical issue when considering application of such tools in this population, also remains poorly addressed. The current review addresses this knowledge gap by conducting a comprehensive review of the psychometric properties and feasibility of using PA self-report tools in older adults with MCC. The attained results will help inform treating clinicians and older adults with MCC who are engaged in health management strategies about the utility of these tools.
The Review
Aim
The aim of this study was to address the knowledge gaps described, through identification of the self-report PA tool that would be best suited for the assessment of PA in older adults with MCC. This was achieved through performing a review that reported on: (1) the psychometric properties (reliability and validity) of PA self-report tools applied to older adults and (2) the feasibility of using PA self-report tools in older adults with MCC.
Design
The design of this review is that of a systematic review (Aromataris & Pearson, Reference Aromataris and Pearson2014). After development of a clear statement of research aims, inclusion and exclusion criteria were established. Then, a comprehensive search of published studies was conducted applying explicit search terms. The articles captured were assessed for quality (Fayers & Machin, Reference Fayers, Machin, Fayers and Machin2007; Voukelatos et al., Reference Voukelatos, Merom, Rissel, Sherrington, Watson and Waller2011). Articles of sufficient quality were selected, and data were extracted and then analyzed. Finally, data about various study aspects were presented and synthesized leading to recommendations.
Search Methods
The searched databases were: Cochrane Library, Web of Science, PubMed/MEDLINE®, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and AgeLine. The literature search focused on the period from January 2000 to April 2018, to maximize inclusion of relevant articles. The search terms included: physical activity, measure, assess, quantify, evaluate, tool, instrument, survey, questionnaire, older, elder, retiree, valid, verify, consistent, reliable, property, feasible and community (terms were combined with Boolean operators and equivalent terms searched using wild cards) (Appendix 1).
The search was limited to English language articles reporting on the validity and/or reliability and/or feasibility of the self-report PA tools and that only included subjects ≥ 65 years of age who resided at home or in an unassisted living retirement home. Sixty-five years was chosen as the cut-off for inclusion in the review because it is a commonly used and accepted benchmark of the beginning of older adulthood (Orimo et al., Reference Orimo, Ito, Suzuki, Araki, Hosoi and Sawabe2006). Articles were excluded if they reported on people residing in long-term care, assisted living settings, or chronic care facilities, or reported on studies that used diaries or journals rather than validated tools to report PA (Appendix 2).
Search Outcome
One reviewer assessed the abstracts and full-text articles for study inclusion. Reference lists of selected articles were hand searched for additional key sources. A total of 1,702 articles were identified through the database searches, with 1,487 remaining after duplicates (n = 215) were removed. A total of 1,402 articles were removed because they did not address PA in community-dwelling older adults or did not evaluate a PA tool or did not include a focus on PA assessment. A total of 85 full-text articles were assessed for study eligibility, with the remaining articles (n = 66) removed because they did not meet the age inclusion criteria (≥ 65 years) or were not self-report PA tools. A total of 19 studies reporting on 18 self-report PA tools met the inclusion criteria and were included in the review (Figure 1).
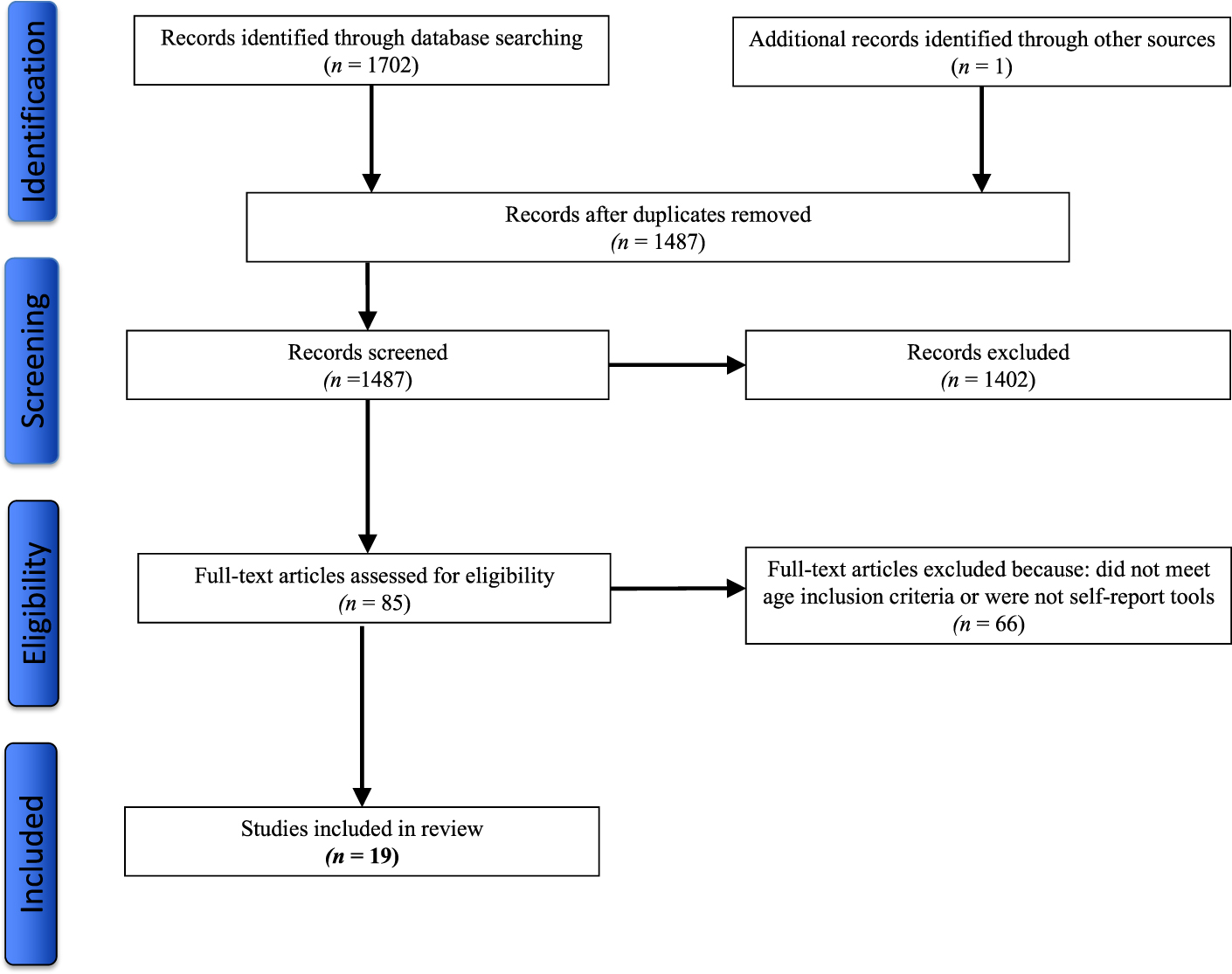
Figure 1: Process of article selection for methodological assessment.
Quality Appraisal
A quality appraisal was conducted by one reviewer on all 19 articles using assessment tools adapted from Fayers and Machin (Reference Fayers, Machin, Fayers and Machin2007) and Voukelatos et al. (Reference Voukelatos, Merom, Rissel, Sherrington, Watson and Waller2011). All articles were appraised using these criteria, and were deemed of suitable quality and included in this review (Table 1).
Table 1: Critical appraisal of included articles

Data Abstraction
Psychometric Properties
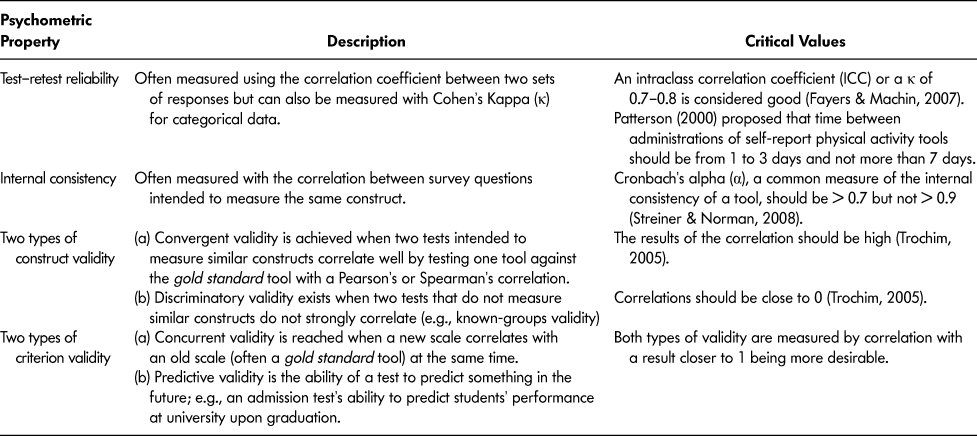
The 19 selected articles reported on 18 self-report PA tools. The properties of these tools were reported on using the methodological framework by Streiner and Norman (Reference Streiner and Norman2008) for the following psychometric properties: test–re-test reliability, internal consistency, content validity, construct validity (convergent and discriminatory validity), and criterion validity (concurrent and predictive validity) (Table 2). Although information for all of these properties was sought, it was not available for all self-report PA tools. All tools reported on at least some psychometric properties.
Table 2: Description of psychometric properties used to assess self-report physical activity tools included in this review
Feasibility
Feasibility is the assessment of whether a plan, activity, or method is practical to implement (Pearson, Field, & Jordan, Reference Pearson, Field and Jordan2007). For the purpose of this review, feasibility refers to the subjective and objective assessment of the practicality of administering self-report PA tools to older adults with MCC. None of the reviewed articles formally assessed self-report PA tools for feasibility; therefore, the authors conducted an assessment of self-report PA tool feasibility for use in older adults with MCC. Two self-report PA tools were not assessed because the authors were unable to access these tools, although the tool creators and/or recent tool users were contacted. The assessment of PA tool feasibility entailed considering common PA restrictions present in older adults with MCC (e.g., types and intensity of PA), as well as other factors such as the older adults’ cognitive status and general wellness, which could affect their ability to complete the self-report tools.
The study authors assessed the feasibility of use of the tools with the following criteria developed by Fayers and Machin (Reference Fayers, Machin, Fayers and Machin2007) and Terwee et al. (Reference Terwee, Mokkink, van Poppel, Chinapaw, van Mechelen and de Vet2010): (1) Is the tool administered by the respondent or by an interviewer (e.g., health professional)? (2) What is the time required to complete the tool? (3) How many questions are in the tool? (4) Who is the intended population for the tool’s application? (5) Are there difficult items in the tool (e.g., additional assistance is necessary to clarify questions, or mistakes are frequently found in completed self-report tools)? (6) Is the tool relevant to the population of interest? (7) Can the tool be completed by a substitute or proxy (e.g., when a respondent is unable to see or write adequately)? (8) What is the recall period of the tool? (9) Does the tool contain integrated instructions for the person completing it? The articles provided some of the information used to assess a tool’s feasibility. However, in other cases articles, provided little or no information and the authors of this review accessed the tool directly to enable assessment of tool feasibility.
Synthesis
Many of the articles on the self-report tools reported on multiple different psychometric properties. In order to best collate and present this information, a decision was made to focus on assessments of psychometric properties that pertained to a broad range of PA types, including low-intensity PA. This enabled the widest applicability of the findings to the older adult population, which may engage in limited amounts of PA. Simultaneously, special attention was given to assessment types that occurred across multiple studies and on gold standard measures such as DLW.
The abstracted data on psychometric properties and of feasibility of the 18 self-report PA tools were collated in tables. The available evidence for tools’ reliability and validity was reviewed across articles to come to an overall assessment of the tools’ psychometric properties and then compared among tools. These comparisons led to the identification of PA tools with the strongest evidence for highest reliability and validity. Subsequently, the available information for tools’ feasibility was reviewed and used to identify the tool(s) with highest feasibility. In cases in which the studies provided limited to no information on feasibility, predetermined criteria were used to evaluate the tools’ feasibility. In the final step, information on tools’ reliability, validity, and feasibility was combined to come to an overall recommendation for the self-report PA tool best suited for application in older adults with MCC.
Results
The 19 articles included in this review provided information on the psychometric properties and feasibility of 18 self-report PA tools (Table 3). Ten of these tools were originally designed for older adults. The remaining eight tools were designed for application to adults (persons ≥ 18 years of age) but applied to older adults. Most of the tools (n = 14) were assessed in only one study; one tool, the Incidental and Planned Exercise Questionnaire (IPEQ) was assessed in two studies; another tool, the YPAS, was assessed in three studies; and two tools, the CHAMPS and the PASE, each were assessed in seven studies (Table 4).
Table 3: Description of 18 self-report physical activity (PA) tools included in this review

Note. aCanadian veterans of World War II/the Korean War or their caregivers; bReferring to all possible settings; cLevel of activity, not further specified; dUnits not further specified.
Table 4: Psychometric properties of 18 self-report PA tools included in this review as reported by 19 reviewed articles

Note. ns p ≥ 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, §p not reported.
a Sample size, gender, age, ethnicity, PA ability. bFor explanation of tool acronyms see Table 2. cStudy also reviewed in Forsen et al. (Reference Forsen, Loland, Vuillemin, Chinapaw, Van Poppel and Mokkink2010). dStudy includes subjects with multiple chronic conditions. eReferent group. fTime frame not reported.
AAS = Active Australia Survey; AAHPERD = American Alliance for Health, Physical Education, Recreation and Dance Functional Fitness Assessment; ACC = accelerometer; Act =Actigraph Accelerometer; APA = absolute physical activity question; CAQ = College Alumni Questionnaire; CHAMPS = Community Healthy Activities Model Program for Seniors; CI = confidence interval; DLW/RMR/PAI = doubly labeled water/resting metabolic rate/physical activity index; DLW TEE = doubly labeled water total energy expenditure; HR = heart rate; ICC = intraclass correlation coefficient; interRAI = interRAI Community Health Assessment Form; IPEQ = Incidental and Planned Exercise Questionnaire; JALSPAQ = Japan Arteriosclerosis Longitudinal Study Physical Activity Questionnaire; K = Cohen’s kappa; LRCQ = Lipid Research Clinics Questionnaire; MBQ = Modified Baecke Questionnaire; MDQ = Modified Dalloso Questionnaire; MLTPAQ = Minnesota Leisure Time Physical Activity Questionnaire; PA = physical activity; PAQ-EJ = Physical Activity Questionnaire, Elderly Japanese; PASE = Physical Activity Assessment Scale for the Elderly; PED = pedometer; PH = perceived health; RPA = relative physical activity question; SDR = Seven Day Recall; SF-12phys = Short-form 12 Health Survey questionnaire, physical activity component; SF-36phys = Short-form 36 physical activity component; SF-36role phys = Short-form 36 role physical; SFT = Senior Fitness Test; SR-PA = Single Self-Report Physical Activity Question; ST = step test; SUAQ = Stanford Usual Activity Questionnaire; TEE/RMR = total energy expenditure by resting metabolic rate; VO2max = maximal oxygen uptake; YPAS = Yale Physical Activity Survey.
Reliability
Eleven of the 19 articles reported on test–re-test reliability of the tools using four different measures (Table 4): (1) nine articles used intraclass correlation (ICC); (2) two used Cohen’s Kappa coefficient (κ); and (3) one used Pearson’s correlation coefficient (r). A total of seven tools were assessed for test–re-test reliability, whereas 11 were not (Table 4).
The reported ICC ranged from 0.75 to 0.91 for studies that used a three to seven-day test–re-test time frame and ranged from 0.60 to 0.78 for test–re-test time frames longer than seven days (Table 4). For the shorter test–re-test time frames, the lowest ICC was found for CHAMPS, with a re-test time frame of seven days (ICC = 0.75), whereas the highest ICC was found for PASE, with a test–re-test time frame of three days (ICC = 0.91). For the longer test–re-test time frames (longer than seven days), PASE and CHAMPS were virtually tied for lowest ICC (PASE: ICC = 0.60, test–re-test time frame = 10 days; CHAMPS: ICC = 0.62, test–re-test time frame = 21–49 days). The highest ICC for the longer test–re-test timeframe was found for CHAMPS (ICC = 0.93), with a test–re-test time frame of 7–14 days (Table 4).
Results for κ ranged from 0.56 for the Relative Physical Activity (RPA) tool with a test–re-test time frame of 33–37 days, to 0.75 for the Absolute Physical Activity (APA) tool with a test–re-test time frame of 33–37 days (Table 4). Both articles reported Cohen’s κ for the whole scale total, and only reported unweighted assessments of κ.
Validity
Eighteen of the 19 articles reported on validity using four different measures (Table 4): (1) ten articles reported on convergent validity; (2) seven reported on concurrent validity; (3) one reported on predictive validity; and (4) one reported on known groups validity. Six articles reported on convergent validity using Spearman’s correlation coefficient (ρ), and four articles used Pearson’s correlation coefficient (r). Six articles reported on concurrent validity using Spearman’s correlation coefficient (ρ) and one article used Pearson’s correlation coefficient (r). One article, Rosario et al. (Reference Rosario, Vazquez, Cruz and Ortiz2008) did not report on validity.
Convergent validity assessed with Spearman’s ρ (Table 4) for step counts ranged from 0.21 to 0.57. Both the highest and lowest values were found for CHAMPS (ρ = 0.21–0.57), with intermediate values for PASE (ρ = 0.36–0.43) and the Active Australia Survey (AAS) (ρ = 0.42). Convergent validity assessed with Spearman’s ρ (Table 4) for DLW ranged from 0.07 for the YPAS to 0.64 for the Stanford Usual Activity Questionnaire (SUAQ(vig.)). Both PASE (ρ = 0.20–0.34) and CHAMPS (ρ = 0.28) showed intermediate values. Using other questionnaires to validate the tools resulted in correlations (ρ) of 0.39 for the AAS and 0.10–0.57 for the APA/RPA.
Convergent validity assessed with Pearson’s r (Table 4) for the six-minute walk test ranged from 0.21 to 0.68. The lowest value was found for CHAMPS (r = 0.21) and the highest value was found for PASE (r = 0.68). When compared to step counts, Pearson’s r was 0.35–0.36 for the Zutphen Physical Activity Questionnaire (ZPAQ). Using other questionnaires to validate the tools resulted in correlations (r) ranging from 0.30 for CHAMPS to -0.34 for PASE (Table 4).
Concurrent validity assessed with Spearman’s ρ (Table 4) for PASE ranged from 0.47 to 0.58 when the tool was compared with other questionnaires, and was -0.28 (reverse scale) when compared with walking speed. For IPEQ and the Physical Activity Questionnaire (PAQ), ρ ranged from 0.09 to 0.23 and 0.41, respectively, when compared with accelerometers. For SR-PA, ρ ranged from 0.40 to 0.49, when compared with accelerometers and time spent on low- or moderate-intensity PA.
Feasibility
Sixteen of the 18 tools were available for access allowing an assessment of feasibility aspects for use in older adults with MCC beyond the aspects reported by the reviewed articles (Table 5). The two remaining self-report PA tools (Modified Dalloso Questionnaire [MDQ] and Questionnaire d’Activité Physique Saint-Etienne [QAPSE]) could not be accessed despite attempts to make contact with the tool creators and/or recent tool users (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). Because this lack of access resulted in very incomplete information, these tools had to be excluded from further feasibility assessment.
Table 5: Feasibility aspects of 18 self-report PA tools included in this review

Note. aFor explanation of tool acronyms see Table 3 bQuestions cover all activities of daily life not just formalized PA. cQuestionnaire can be completed by another person on one’s behalf. dUnable to gain access to these tools or information about them, therefore feasibility aspects of these tools could not be assessed. eCompared with previous 3 months. f17 main items but 29 items when counting sub-items.
Seven of the reviewed self-report PA tools are interviewer-administered, six are self-administered, and three can be either self-administered or interviewer-administered (Table 5). The time required for completing the tools ranged from less than 5 minutes for the APA, Lipid Research Clinics Questionnaire (LRCQ), RPA, and SR-PA to more than 30 minutes for the Minnesota Leisure Time Physical Activity Questionnaire (MLTPAQ) (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Gill, Jones, Zou, & Speechley, Reference Gill, Jones, Zou and Speechley2012; Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017) . The number of questions per tool ranged from 1 (SR-PA) to 63 (MLTPAQ) (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017). Eight tools contained some questions that were difficult to answer by study participants as reported by the article authors (Table 5). Two tools (LRCQ and SUAQ) were not relevant to older adults because they did not address activities of daily life (e.g., housework or gardening) that are important in this population (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). Most articles (n = 13) did not specify whether the tools could be completed by a substitute for the intended subject, whereas three stated explicitly that a substitute was not permitted (Table 5). Six tools had a recall time frame of one week, whereas the recall time frame of the remaining tools ranged from four weeks to 12 months (Table 5).
Discussion
To our knowledge, this is the first systematic review reporting on the reliability, validity, and feasibility of self-report PA tools for community-dwelling older adults with MCC, with the aim of identifying the most suitable tool for use in this population. Overall, 19 articles were reviewed reporting on 18 tools that were assessed for reliability, validity, and feasibility.
Psychometric Properties
Self-report PA tools must undergo rigorous evaluation of their psychometric properties in order to have meaningful application to inform research and clinical practice. These tools must be reliable and valid in their ability to detect PA in the population of interest: in this case, older adults with MCC. However, this review determined that reliability was reported on less than half of the self-report PA tools. All articles reporting ICC as a measure of reliability indicated moderate to excellent reliability for the tools they tested. PASE and CHAMPS were found to have the highest reliability, and both were reported to have excellent or close to excellent reliability (Koo & Li, Reference Koo and Li2016). However, a range of factors including the exact model used to calculate this statistic affects ICC values (Koo & Li, Reference Koo and Li2016; Lee et al., Reference Lee, Lee, Chung, Ahn, Sung and Kim2012). Unfortunately, only one of the reviewed articles provided information about the exact ICC model used (for IPEQ), making comparison of reliability among the various tools more difficult (Delbaere, Hauer, & Lord, Reference Delbaere, Hauer and Lord2010).
Information on convergent validity was provided for 15 of the self-report PA tools. The results suggest that the six tools showing highest convergent validity (≥ 0.50 for: CHAMPS, PASE, RPA, Seven Day Recall [SDR], SUAQ, YPAS) strongly overlap in their validity values. CHAMPS, PASE and YPAS were assessed most often for convergent validity, providing greater evidence for their validity, but showing a wide range of values for all three tools (Table 4). Convergent validity for all three tools tended to be higher when the tools were compared with direct behavioural measures (e.g. an accelerometer) than compared with direct metabolic measures (e.g., DLW) (Colbert, Matthews, Havighurst, Kim, & Schoeller, Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Harada, Chiu, King, & Stewart, Reference Harada, Chiu, King and Stewart2001; Ngai, Cheung, Lam, Chiu, & Fung, Reference Ngai, Cheung, Lam, Chiu and Fung2012).
Information on concurrent validity was provided for five self-report PA tools. Concurrent validity for PASE and IPEQ was reported by two articles, but was reported by only one article for the SR-PA, Physical Activity Questionnaire – Elderly Japanese (PAQ-EJ) and CHAMPS, thus making available more evidence for validity assessment of PASE and IPEQ. By far the highest values for concurrent validity were found for IPEQ (Delbaere et al., Reference Delbaere, Hauer and Lord2010). However, these values were found by comparing two versions of the IPEQ with each other, instead of with another independent tool. When comparing the IPEQ with PA measured with accelerometers, concurrent validity was much lower (Merom et al., Reference Merom, Delbaere, Cumming, Voukelatos, Rissel and Van Der Ploeg2014). Excluding the previously mentioned concurrent validity values for IPEQ, the highest and most consistent validity values were found for PASE (Hagiwara, Ito, Sawai, & Kazuma, Reference Hagiwara, Ito, Sawai and Kazuma2008; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012). Overall, the evidence for moderate to high concurrent validity was strongest for PASE.
Particular areas of concern regarding self-report PA tools relate to their ability to detect change in health status over time. The current review found few studies that assessed the tools’ sensitivity to change over time, and those that did used variable time frames and comparison modalities, making it difficult to compare and contrast across studies (Godard & Standley, Reference Godard and Standley2006; Harada et al., Reference Harada, Chiu, King and Stewart2001; Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017; Stewart et al., Reference Stewart, Mills, King, Haskell, Gillis and Ritter2001). Although designed with older adults in mind, CHAMPS was shown to be less sensitive to lighter PA levels, limiting its suitability for older adults with MCC (Godard & Standley, Reference Godard and Standley2006).
Feasibility in Older Adults with MCC
The feasibility assessment revealed that a number of self-report PA tools (e.g., PASE and ZPAQ) stood out for generally more favourable feasibility aspects, such as taking less time to administer and requiring a shorter recall period. These tools were deemed by us to be more feasible for use with older adults with MCC than the other assessed tools.
Only five of the reviewed articles explicitly stated that they included older adults with MCC (but excluding cognitive conditions) in their studies (Cyarto, Marshall, Dickinson, & Brown, Reference Cyarto, Marshall, Dickinson and Brown2006; Gill et al., Reference Gill, Jones, Zou and Speechley2012; Harris et al., Reference Harris, Owen, Victor, Adams, Ekelund and Cook2009; Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017; Stewart et al., Reference Stewart, Mills, King, Haskell, Gillis and Ritter2001). Some articles specified that subjects had to be sufficiently physically healthy to engage in PA such as walking, and all articles stated that subjects had to be free of major cognitive conditions that would affect their ability to self-report PA (Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Giles & Marshall, Reference Giles and Marshall2009; Merom et al., Reference Merom, Delbaere, Cumming, Voukelatos, Rissel and Van Der Ploeg2014;). Although it can be assumed that the ability to respond appropriately to self-report PA tools is limited by major cognitive impairment, it is less clear whether this is also always the case for mild cognitive impairment. Everyday function in people with mild cognitive decline may be comparable to that of a control group, although everyday memory recall may be reduced and become worse with increased recall period (Anstey, Eramudugolla, Chopra, Price, & Wood, Reference Anstey, Eramudugolla, Chopra, Price and Wood2017; Irish, Lawlor, Coen & O’Mara, Reference Irish, Lawlor, Coen and O’Mara2011). Because the prevalence of mild cognitive impairment in the older adult population may be as high as 42 per cent, the use of self-report PA tools under conditions of mild cognitive impairment requires further study (Tricco et al., Reference Tricco, Soobiah, Lillie, Perrier, Chen and Hemmelgarn2012).
In addition to limitations in engaging in PA (Harada et al., Reference Harada, Chiu, King and Stewart2001), older adults with MCC may face physical limitations such as arthritis and declining visual acuity (diabetes related or age related) (Anderson & Horvath, Reference Anderson and Horvath2004; Canadian Institute for Health Information, 2011; Harada et al., Reference Harada, Chiu, King and Stewart2001), which could influence their ability to complete a self-report PA tool. One study using PASE reported that a small proportion of subjects needed additional explanations and assistance to complete the questionnaire (Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008). Cyarto et al. (Reference Cyarto, Marshall, Dickinson and Brown2006) determined that 25 per cent of participants required assistance with CHAMPS, whereas a mail survey study by Giles and Marshall (Reference Giles and Marshall2009) found that only 60 per cent of returned CHAMPS questionnaires were fully completed. Factors such as enabling completion of the self-report PA tool by a support person, simple wording, and training older adults in the use of the tools may help improve the feasibility of using PA self-report tools in the older adult population.
The length of the recall period is an important aspect of self-report PA tools. Shephard (Reference Shephard2003) suggests that recall diminishes with the length of the recall period. However, some of the reviewed self-report PA tools required recall of up to 12 months, with the College Alumni Questionnaire (CAQ) requiring recall of daily, weekly and yearly activities in increments of hours and minutes (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). This recall demand would be challenging for people of all ages, but perhaps particularly so for older adults with MCC who may not engage in regular, regimented schedules of activity.
From a feasibility perspective, two tools, the ZPAQ and PASE stood out for having high feasibility (Bonnefoy et al, Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger, Oman, Taylor, Vesely, & Able, Reference Dinger, Oman, Taylor, Vesely and Able2004; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Harris et al., Reference Harris, Owen, Victor, Adams, Ekelund and Cook2009; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). The ZPAQ may be a very feasible tool overall for older adults with MCC. It is self-administered, contains 17 questions (29 when including sub-items), is expected to take 15 minutes to complete, was designed to capture the PA of older adults 65 years or age or more, and has a recall period of one month. However, PASE may have even higher feasibility as it can be self- or interviewer-administered, contains just 10 questions, takes only five minutes to complete, was designed for use in older adults 65 years of age or more, and has a recall period of only one week. Although the CHAMPS self-report tool was also specifically developed for older adults, the fact that it contains 40 items and has a recall period of one month may make it less feasible for older adults with MCC (Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Cyarto et al., Reference Cyarto, Marshall, Dickinson and Brown2006; Giles & Marshall, Reference Giles and Marshall2009; Godard & Standley, Reference Godard and Standley2006; Harada et al., Reference Harada, Chiu, King and Stewart2001; Rosario et al., Reference Rosario, Vazquez, Cruz and Ortiz2008; Stewart et al., Reference Stewart, Mills, King, Haskell, Gillis and Ritter2001).
Self-Report PA Tool Recommendation
Following evaluation, some self-report PA tools emerged as most suitable for older adults with MCC. The SUAQ was highlighted by Bonnefoy et al. (Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001) because it correlated well with DLW. However, the participants in that study were healthy males who were capable of engaging in maximal physical exertion and had no serious illnesses. Because the SUAQ was designed to report moderate to vigorous PA, it may not be surprising that it correlates well with DLW (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). However, whether the SUAQ is suitable to capture the low-intensity PA typical of older adults with MCC is unclear (Bonnefoy et al. Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001). Although PASE does not correlate well with direct measures of energy expenditure such as DLW, it does correlate well with accelerometers and walking ability (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Hagiwara, et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). This makes it well suited for older adults with health constraints who are most likely to engage in low-intensity PA. PASE was also determined to be sensitive to change after a six-week physician counselling intervention, making it well placed for use as part of a health management plan aimed at assisting older adults with MCC in managing their health (Marcus et al., Reference Marcus, Goldstein, Jette, Simkin-Silverman, Pinto and Milan1997).
Both PASE and CHAMPS demonstrated better psychometric properties than other self-report PA tools included in this review. Both tools were shown to have good reliability by at least some studies (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Cyarto et al., Reference Cyarto, Marshall, Dickinson and Brown2006; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Giles & Marshall, Reference Giles and Marshall2009; Godard & Standley, Reference Godard and Standley2006; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Rosario et al., Reference Rosario, Vazquez, Cruz and Ortiz2008; Stewart et al., Reference Stewart, Mills, King, Haskell, Gillis and Ritter2001; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). However, PASE displayed better concurrent validity than CHAMPS (Godard & Standley, Reference Godard and Standley2006; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012). In most cases, PASE also displayed convergent validity comparable to or better than CHAMPS (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Giles & Marshall, Reference Giles and Marshall2009; Harada et al., Reference Harada, Chiu, King and Stewart2001; Stewart et al., Reference Stewart, Mills, King, Haskell, Gillis and Ritter2001; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). Additionally, PASE rated better than CHAMPS in terms of feasibility, with just 10 questions and a one-week recall period compared with the 40 questions and a four-week recall period for CHAMPS (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). Although ZPAQ and PASE are rated similarly high in feasibility, there is not enough evidence available for comprehensive assessment of the ZPAQ’s psychometric properties (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Harris et al., Reference Harris, Owen, Victor, Adams, Ekelund and Cook2009; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). ZPAQ has also been criticized for not containing any questions pertaining to household activities, which are an important source of PA in older adults (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Hagiwara et al., Reference Hagiwara, Ito, Sawai and Kazuma2008; Harada et al., Reference Harada, Chiu, King and Stewart2001; Harris et al., Reference Harris, Owen, Victor, Adams, Ekelund and Cook2009; Ngai et al., Reference Ngai, Cheung, Lam, Chiu and Fung2012; Washburn et al., Reference Washburn, Smith, Jette and Janney1993). Based on the results of this review, the main recommendation is that PASE is the most appropriate self-report tool to assess PA in older adults with MCC. This recommendation is congruent with the use of PASE as the PA data collection tool for the Canadian Longitudinal Study on Aging (Canadian Longitudinal Study on Aging, 2015).
Relevance to Health Practice and Research
Increasingly greater numbers of older adults with MCC are living independently. Evidence shows that independence in older age benefits their social networks and overall health (Wiles et al., Reference Wiles, Leibing, Guberman, Reeve and Allen2012). Moreover, PA has demonstrated success in helping alleviate the risk of falls or further health decline associated with aging (Schnitzer et al., Reference Schnitzer, Blüher, Teti, Schaeffner, Ebert and Martus2019). Health professionals, such as nurses, are directly involved in supporting older adults with MCC in managing their health using strategies such as ongoing health monitoring and administration of care plans that increasingly include regular exercise (Rippe, Reference Rippe2018). In this context, tools for reporting of PA are needed with acceptable levels of reliability and validity that are feasible for use in older adults with MCC. Use of such tools should enable health professionals to monitor their clients’ progress and changes in health, and measure whether health outcomes have been achieved (Forsen et al., Reference Forsen, Loland, Vuillemin, Chinapaw, Van Poppel and Mokkink2010). Self-report PA tools are inexpensive, easily utilized, and often well suited to assessing a broad range of PA (Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Helmerhorst, Brage, Warren, Besson, & Ekelund, Reference Helmerhorst, Brage, Warren, Besson and Ekelund2012). Consequently, simple, easily administered, and reliable self-report PA tools such as PASE have an important role to play in supporting positive health behaviours and managing chronic conditions in older adults with MCC. These tools also have relevant applications in research such as assessing relative changes in PA levels over time at the population level and in making comparisons among populations (Portegijs et al., Reference Portegijs, Sipilä, Viljanen, Rantakokko and Rantanen2017).
Strengths and Limitations
Strengths of this review include its extensive coverage of the literature through a comprehensive search of the literature extending from 2000 through 2018. Multiple databases were used to ensure broad representation of the available literature. Conducting an assessment of self-report PA tool feasibility in community-dwelling older adults with MCC, in addition to assessing their validity and reliability, makes this review particularly relevant for informing clinical practice and research.
Limitations of the review were the inclusion of only English articles, which may have limited the diversity and global representativeness of included articles. Further, one reviewer conducted the literature search, data extraction, and evaluation, whereas in ideal circumstances, there would be at least two reviewers. The exclusion of two articles from the feasibility assessment of the PA tools because of an inability to obtain the tools is also a shortcoming of the review.
Overall, articles included in this review were lacking in study populations that were characteristic of older adults with MCC. Often samples consisted of convenience samples with unequal gender balances or only healthy males (Bonnefoy et al., Reference Bonnefoy, Normand, Pachiaudi, Lacour, Laville and Kostka2001; Colbert et al., Reference Colbert, Matthews, Havighurst, Kim and Schoeller2011; Cyarto et al., Reference Cyarto, Marshall, Dickinson and Brown2006; Dinger et al., Reference Dinger, Oman, Taylor, Vesely and Able2004; Gill et al., Reference Gill, Jones, Zou and Speechley2012; Harada et al., Reference Harada, Chiu, King and Stewart2001; Merom et al., Reference Merom, Delbaere, Cumming, Voukelatos, Rissel and Van Der Ploeg2014). Going forward, research is required that includes study samples that are more representative in gender and health, with direct attention given to the systematic study of feasibility and sensitivity to clinically relevant changes in health status in older adults with MCC. Furthermore, many of the tools were not comprehensively assessed for their psychometric properties, requiring further study of self-report PA tools to close this knowledge gap.
Other questions that require further study include asking under which conditions self-report PA tools might be used successfully by older adults with mild cognitive impairment, what the effects are of various chronic conditions on older adults’ ability to complete self-report PA, and what is the most suitable recall period in older adults for the use of self-report PA tools.
Conclusion
Eighteen self-report PA tools were reviewed for their psychometric properties and feasibility for use in assessing PA in older adults with MCC. All tools for which reliability was reported (n = 7) showed good to excellent reliability in at least some tests. Although reported validity ratings were variable, assessed self-report PA tools (n = 18) consistently compared more favourably with other questionnaires and direct behavioural measures (i.e., an accelerometer) than with direct metabolic measures. Although some studies explicitly included older adults with MCC in their study populations, there remains a dearth of research available on the psychometric properties and feasibility of self-report PA tools for assessing PA in older adults with MCC. Further research that includes older adults with MCC is warranted to understand the utility of these tools for this population. When feasibility was accounted for, the available evidence suggests that PASE is the most suitable self-report PA tool to assess PA in older adults with MCC.
Appendix 1
Search Strategy - MEDLINE®

Appendix 2
Inclusion and exclusion criteria for studies included in this review











