CVD are the main causes of death, currently representing 31 % of all deaths in the world(1) and 27 % of all deaths in Brazil(2). Studies have revealed the presence of modifiable risk factors in adolescence that influence the development of CVD in adult life, such as overweight, dyslipidaemia, diabetes mellitus, physical inactivity, inadequate eating habits, among others(Reference Lauer, Connor and Leaverton3–Reference Raitakari, Juonala and Rönnemaa5).
Adolescence is a transition phase between childhood and adulthood, and it is marked by rapid changes in size, shape and body composition, which occur mainly during puberty. These changes are mediated by hormones, including the growth hormone, the increased production of which induces insulin resistance (IR)(Reference Moran, Jacobs and Steinberger6). The excessive accumulation of body fat, especially in the abdominal region, is associated with the increased secretion of peptides and fatty acids that are responsible for metabolic changes, contributing to the development of IR(Reference Golay7). With the increased prevalence of overweight among Brazilian adolescents(Reference Cureau, Silva and Bloch8,9) , therefore, the physiological character of IR in adolescent puberty may progress pathologically, increasing the risk of glucose intolerance or even causing metabolic syndrome(Reference Yin, Li and Xu10).
Laboratory exams for the diagnosis of IR are invasive and costly, especially for population tracking studies(Reference Jensen, Camargo and Bergamaschi11). As such, anthropometric parameters are important alternatives to overcome these limitations(Reference Vázquez-Vela, Torres and Tovar12). BMI and waist circumference (WC) are the most frequently used measures to assess obesity and cardiovascular risk, but the waist:height ratio (WHtR) has been considered to be easy to apply and interpret, in addition to having a single cut-off point(Reference Lo, Wong and Khalechelvam13), in contrast to the percentile classification of the other parameters(Reference Fernández, Redden and Pietrobelli14–16).
The cut-off point of 0·5 for WHtR is used to predict cardiometabolic risk factors, both in adults and in children or adolescents(Reference Lo, Wong and Khalechelvam13,Reference Ashwell, Gunn and Gibson17,Reference Yoo18) . However, no study considered the adolescents’ stage of sexual maturation, a period of important metabolic oscillations and changes in body measurement, and it is reasonable to assume that this may not be the best cut-off point to be used in adolescents.
As such, the objective of this study was to verify if the WHtR cut-off point used is indeed the most suitable for identifying IR in adolescents and if this value changes depending on the sexual maturation stage.
Methods
This study is characterised as a cross-sectional epidemiological study based on data from the ‘Estudo de Riscos Cardiovasculares em Adolescentes – ERICA’ (Study of Cardiovascular Risks in Adolescents). ERICA is a cross-sectional, multicentre, national study of the school base with the objective of estimating the prevalence of cardiovascular risk factors and metabolic syndrome, in a representative sample of adolescents between 12 and 17 years of age, students of public or private schools from all over Brazil.
The following baseline parameters were considered for the calculation of the sample size of the ERICA study: prevalence of metabolic syndrome in adolescents of 4 %, with maximum absolute error of 1 % and confidence level of 95 %. A simple random sample size would therefore be estimated at 1475 adolescents. Since the sample is grouped by school and class, a design effect of 3 was used, which resulted in a sample size of 4425 adolescents. However, considering that the precision-controlled estimates had to be produced for twelve domains (six ages × two genders), it was estimated that the total sample size would have to be 74 340 individuals(Reference Bloch, Szklo and Kuschnir19,Reference Vasconcellos, Silva and Szklo20) .
The data were collected in 2013 and 2014. In total, 74 589 adolescents from 124 municipalities participated in the survey. The survey population was stratified into thirty-two geographical strata, made up of the twenty-seven capitals and five strata made up of sets of municipalities with more than 100 000 inhabitants in each of the five Brazilian macro-regions(Reference Bloch, Szklo and Kuschnir19,Reference Vasconcellos, Silva and Szklo20) . The field team was comprised by health professionals who were previously trained in standardisation and data collection quality(Reference Bloch, Szklo and Kuschnir19).
A representative sample was obtained on the national, regional and municipal levels and involved three selection stages (school, class and students) with probability proportional to the universe. All eligible adolescents who signed the consent form participated in the data collection. Excluded from this study were those adolescents who did not belong to the age group (12–17 years old), pregnant adolescents, adolescents with some lower limb impairment that makes it difficult to stand alone, as well as those using an external prosthesis or with a mental impairment. Previous publications have described the details about the protocol, design and sampling of the study(Reference Bloch, Szklo and Kuschnir19,Reference Vasconcellos, Silva and Szklo20) .
The anthropometric measurements (height and WC) were taken with the individuals wearing light clothes and no shoes. The weight was obtained with a digital scale (model P150m, 200 kg capacity and 50 g accuracy, Líder®, São Paulo, Brazil) in a single measurement due to the accuracy of the scale. Height was measured to the nearest 1 mm using a calibrated stadiometer (portable stadiometer Alturexata®, Minas Gerais, Brazil) with millimeter resolution and height up to 213 cm. The subjects were in full standing position (in the Frankfort horizontal plane). For quality control purposes, the measurements were made in duplicate, allowing for a maximum variation of 0·5 cm between the two measurements(Reference Yoo18,Reference Bloch, Szklo and Kuschnir19) .
Central adiposity was measured through WC, using an anthropometric glass fibre tape with a millimetric resolution and a length of 1·5 meters (Sanny®, São Paulo, Brazil). The subjects were in vertical position with the abdomen relaxed, and the measurement was taken horizontally at the midpoint between the iliac crest and the lower costal margin, at the end of a soft expiration(Reference Bloch, Szklo and Kuschnir19). The WHtR was calculated with the WC and height data by dividing WC (in cm) by height (in cm).
The blood samples were taken by qualified professionals in the schools participating in the study, in a sub-sample of 40 thousand adolescents. The participants were instructed to fast overnight for 12 h before the exam. Blood samples were processed, and the plasma and serum separated within two hours of collection and kept between 4°C and 10°C while they were transferred to the study’s single laboratory.
The IR index was estimated with the homeostasis model assessment-estimated insulin resistance (HOMA-IR) method based on the equation HOMA-IR = Fasting Insulinaemia (mU/l) × Fasting Blood Sugar (mmol/l)/22·5. IR was considered when the HOMA-IR exceeded 3,16(21,Reference Keskin, Kurtoglu and Kendirci22) .
Two questionnaires were applied to obtain the socio-economic-demographic, lifestyle and health variables: one for the adolescents, one for the caretakers and another for the school environment. The first questionnaire was answered by the adolescents themselves in the Personal Digital Assistant (PDA) instrument. It was made up of questions related to socio-demographic aspects, work, physical activity, diet, smoking, alcoholic beverages, reproductive health, oral health, referred morbidity, sleep and mood/disposition. The questionnaire of the caretakers covered questions about their identification and the adolescent’s health.
The evaluation of sexual maturation was performed through self-assessment using Tanner’s boards(Reference Tanner23). Participants identified, among the images presented on the Tanner board, with each stage of maturation (from the breasts – M1 to M5, penis and testes – G1 to G5, where G1 was pre-pubertal, G2–G4, pubertal and G5 post-pubertal) for boys, and these indicated that image that corresponded, with greater precision, to the current stage of their own sexual maturation(Reference Tanner23).
The adolescents were distributed according to sex and stage of sexual maturation(Reference Tanner23) and later classified, for each stratum, in accordance with the IR. An Receiver Operating characteristic (ROC) curve was constructed for each group in order to determine the best WHtR cut-off points for the sample stratified by sex and stratified by sex and sexual maturation stage. The areas under the ROC curve and the 95 % CI were used to evaluate the diagnostic value of WHtR. The areas under the curve where evaluated as less accurate (0·5 < AUC 0·7), moderately accurate (0·7 < AUC < 0·9), highly accurate (0·9 < AUC < 1·0) and perfect (AUC = 1·0)(Reference Greiner, Pfeiffer and Smith24,Reference Swets25) . The sensitivity and specificity of the chosen cut-off points were calculated for each case. All analyses were performed with the aid of the statistical package STATA 14·0 se.
The ERICA study was approved by the Research Ethics Committees (CEP) of the Instituto de Estudos em Saúde Coletiva of the Universidade Federal do Rio de Janeiro (Case 45/2008) and by the ethics committees of each Brazilian state. All interviewed and examined adolescents provided the informed consent signed by them and the informed consent form signed by their guardians. The student’s privacy and the confidentiality of the information was guaranteed.
Results
A total of 37 759 adolescents were evaluated, most of them female (60·0 %), with a mean age of 14·7 years (sd ± 0·08 years) and with 13·1 % presenting IR. When stratified by sex, the IR frequency was higher among girls (14·0 % in girls v. 11·6 % in boys).
The girls showed a behaviour of increasing IR frequency in sexual maturation stages 4 and 5 when compared with the other stages: 63·7 % of girls with altered HOMA-IR could be found in these stages. No difference was observed in the altered HOMA-IR frequencies at the different maturation stages of the boys.
The prediction of IR through the WHtR indicator was better for boys, where all the areas under the ROC curve were superior to 0·70 (Fig. 1). In girls, the highest AUC was observed in Tanner stage 5, equal to 0·74 (Fig. 2).
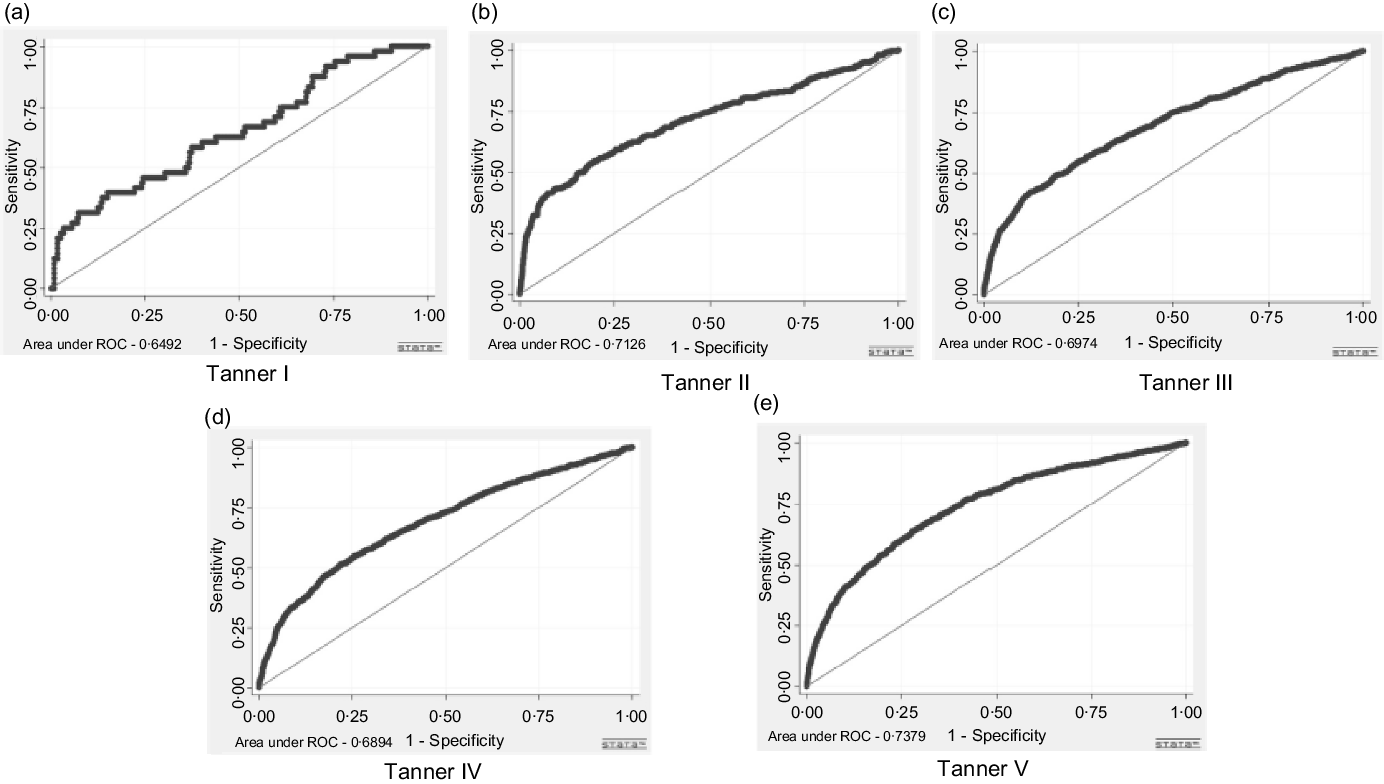
Fig. 1. ROC curves of the waist:height ratio for the prediction of insulin resistance (HOMA-IR) of Brazilian adolescent girls, Brazil, 2013–2014.

Fig. 2. ROC curves of the waist:height ratio for the prediction of insulin resistance (HOMA-IR) of Brazilian adolescent boys, Brazil, 2013–2014.
The best WHtR cut-off points found stratifying only by sex, were equal to 0·45 for girls and 0·44 for boys, i.e. higher values would suggest a greater risk of IR for each sex, respectively (Table 1).
Table 1. Cut-off points in the waist:height ratio, by sex, for the prediction of insulin resistance (HOMA-IR) in Brazilian adolescents, Brazil, 2013–2014 (n 37 759)

CP – cut-off point; AUC – area under the curve; CI – confidence interval; S – sensitivity; SP – specificity.
* Usually recommended cut-off value for the population(Reference Raitakari, Juonala and Rönnemaa5,9,Reference Yin, Li and Xu10) .
When the stratification by Tanner maturation stages is included, the boys showed a behaviour of reduction of the cut-off point as they advanced through the sexual maturation stages, while the girls showed an opposite behaviour (Tables 2 and 3).
Table 2. Optimal cut-off point values, area under the ROC curve and their sensitivity and specificity for the waist:height ratio, regarding the prediction of insulin resistance, in adolescent boys, Brazil, 2013–2014 (n 15 097)

CP – cut-off point; AUC – area under the curve; CI – confidence interval; S – sensitivity; SP – specificity.
* Usually recommended cut-off value for the population(Reference Raitakari, Juonala and Rönnemaa5,9,Reference Yin, Li and Xu10) .
Table 3. Optimal cut-off point values, area under the ROC curve and their sensitivity and specificity for the waist:height ratio, regarding the prediction of insulin resistance, in adolescent girls, Brazil, 2013–2014 (n 22 638)

CP – cut-off point; AUC – area under the curve; CI – confidence interval; S – sensitivity; SP – specificity.
* Usually recommended cut-off value for the population (Reference Raitakari, Juonala and Rönnemaa5,9,Reference Yin, Li and Xu10).
Discussion
In this study, the optimal WHtR cut-off value for the prediction of IR was obtained using the ROC curve technique with data from a large sample of adolescents. The availability of data on anthropometric measurements, biochemical indicators and sexual maturation stages has enabled the first robust study to evaluate these relations in more than 37 thousand Brazilians adolescents.
The results show that the AUC values were higher than 0·5 for both sexes and in all Tanner stages, confirming they were not the result of chance. In boys, all the AUC were greater than 0·7, which demonstrates that the screening with WHtR is a satisfactory test for IR in this group, in all Tanner stages. The results were not as expressive for the girls. These results reaffirm the favourable findings for the use of WHtR as a predictor of metabolic changes associated with cardiovascular risk(Reference Lo, Wong and Khalechelvam13,Reference Ashwell, Gunn and Gibson17,Reference Yoo18,Reference Lee, Park and Yum26) .
The message ‘Keep your waist at less than half your height’, i.e., a cut-off point of 0·5(Reference Yoo18) is recommended for the adult and paediatric population around the world. However, several cut-off points have been found, varying according to race, sex, metabolic outcomes, presence of overall obesity or the age of the participants in the study(Reference Jia, Xu and Ming27–Reference Chen29). In this study, the WHtR cut-off points found by sex were below the observed cut-off points in most previously published studies.
When the predictive ability of WHtR for abdominal or general obesity is evaluated, the cut-off points found in the various countries generally approach the recommended value o 0·5, but new evidence has shown that values lower than 0·5 may be more adequate. Dumith et al. (Reference Dumith, Muraro and Monteiro30) found cut-off points for the diagnosis of excess weight of 0·46 for boys and 0·48 for girls in a city in the northeast of Brazil. Previous studies have proposed similar cut-off values for the screening of obesity, with 0·48 for boys and 0·47 for girls in Australia, respectively,(Reference Nambiar, Hughes and Davies31) and 0·47 for boys and 0·45 for girls in China(Reference Zhou, Yang and Yuan32). In Korea, Choi et al. (Reference Choi, Hur and Kang33) recently found WHtR cut-off points for the prediction of abdominal obesity of 0·50 for boys and 0·48 for girls and of 0·47 for general obesity in both sexes.
In this study, the WHtR cut-off points for predicting metabolic outcomes were 0·45 and 0·44 for girls and boys, respectively. This result is close to that of Hara et al. (Reference Hara, Saitou and Iwata34) in Japan, where the optimal cut-off values for MS prediction were 0·41 and 0·44. They were also similar to those of Choi et al. (Reference Choi, Hur and Kang33) in Korea, who found cut-off points of 0·44 and 0·43 for the diagnosis of MS in boys and girls, respectively. In Africa, the cut-off points were a little higher, 0·465 and 0·455 for boys and girls, respectively(Reference Matsha, Kengne and Yako35). In the USA, on the other hand, a single and higher cut-off point was found compared with the others for the prediction of MS, 0·52 for both sexes(Reference Bauer, Marcus and Ogden36).
Studies that evaluate the predictive capacity of WHtR for IR are scarce. One study conducted with obese adults in Southeastern Brazil found an IR prediction cut-off point of 0·62(Reference Jamar, Almeida and Gagliardi37). A study conducted with Brazilian children found a cut-off point of 0·47 for both sexes(Reference Kuba, Leone and Damiani38). The cut-off points should vary depending on the location of the study as well as the investigated outcome, since there is great racial variety between the populations involved in the studies, and it is known that there are differences in the patterns of body fat distribution among the different ethnicities as well as various effects of visceral fat on metabolic changes, depending on these ethnicities(Reference Wells39). In addition, methodological aspects may also be of influence, such as the different methods of measuring the WC that were used in the studies(Reference Matsha, Kengne and Yako35,Reference Jamar, Almeida and Gagliardi37,Reference Kuba, Leone and Damiani38) .
In a recent meta-analysis, the cut-off point of 0·5 was suggested in the paediatric screening of MS(Reference Lo, Wong and Khalechelvam13). In the present study, we checked the sensitivity and specificity of the cut-off point of 0·5 in the prediction of IR and observed an increase in the specificity of the indicator, consequently reducing its sensitivity. As such, we believe that adopting the single value of 0·5 as the cut-off point for WHtR runs the risk of yielding a very specific, but not very sensitive indicator, which will only be useful in clinical practice, but not for epidemiological cases.
In this case, the lower the WHtR cut-off point, the greater the sensitivity, but it also increases the likelihood of diagnosing healthy adolescents as resistant to insulin, which may result in the assignment of unnecessary resources and treatments to revert the metabolic abnormality(Reference Graves, Garnett and Cowell40). An increase in the cut-off point, on the other hand, increases specificity and leads to an increase of another type of error, which is classifying a teenager with IR as healthy and therefore leaving him out of the risk group at the time of screening(Reference Graves, Garnett and Cowell40).
Given the important role that sensitivity plays in epidemiological studies for the early detection of risk factors for CVD in the population of children and adolescents, a better balance between sensitivity and specificity should be prioritised in the choice of cut-off points. In this sense, the use of a general value of 0·44 (boys, 0·44; girls, 0·45) as a cut-off point is appropriate for the early detection and prevention of IR in Brazilian adolescents, prioritising sensitivity and considering a better sensitivity x specificity ratio than higher cut-off points, such as 0·5.
It should be emphasised that the most interesting aspect of the results presented in this study was the opposite behaviour presented by the sexes when the sexual maturation stage is considered. The cut-off point tended to reduce in adolescent boys with advancing sexual maturity, while the cut-off point in adolescent girls tended to increase.
It has been well established in the literature that there is a physiological and progressive decrease in insulin sensitivity during puberty as the sexual maturation stages evolve, reaching a minimum level in the middle of maturation (Tanner III), and returning to prepubescent levels at the completion of the process (Tanner V)(Reference Hannon, Janosky and Arslanian41,Reference Reinehr, Wolters and Knop42) .
Just as happens with metabolic changes, body composition changes considerably during sexual maturation, occurring in different ways according to sex, with girls experiencing more fat accumulation along the maturation stages, while boys build more lean mass(Reference Siervorgel, Demerath and Schubert43). In our study, the cut-off points changed with advancing maturity, with the highest point being observed in the Tanner I stage in boys and in the Tanner V stage in girls, which also coincides with the early stages of greater accumulation of abdominal fat in these groups. Unfortunately, we did not find studies evaluating the WHtR cut-off points or another abdominal obesity indicator considering the sexual maturation stages, which hindered the comparison of our results.
Knowing that these changes are peculiar to adolescence and that they involve a cascade of hormonal changes common to puberty, our results show that it is essential to assess the metabolic risk of adolescents while considering his/her maturation stage, and the adoption of a single cut-off point is not recommended if one is to reduce the chances of errors of classification.
Faced with these findings, we believe that WHtR can function as an important warning sign for professionals in the evaluation of population groups, serving as a useful tool in the detection of risk groups for changes in IR, and therefore contributing to preventive and therapeutic approaches for vulnerable populations.
One of the limitations of this study is that it had a cross-sectional design, which means we could not precisely establish the predictive power of WHtR in diagnosing IR. Future prospective studies are needed to assess the validity of the obtained cut-off values. The Tanner stage was self-reported, which could imply some bias, but the large sample provides some confidence that the differences between the answers and the real situation of adolescents are reduced. In addition, only breast growth (for girls) and genitalia development (for boys) were considered in order to avoid possible biases caused by the erroneous interpretation of the growth of pubic hair, since it is known that the practice of removing these hairs is common among adolescent girls in Brazil. The expansion factors of the sample were not considered, so the study does not represent the population to present the real prevalence of IR, but the relationships observed in the comparison of the groups with and without IR allow for an estimation, through the ROC curve technique, of the best WHtR cut-off points, in addition to the sensitivity and specificity values of each cut-off point for each stratum.
Conclusion
We can conclude that WHtR is a good predictor of IR, as evaluated by HOMA-IR, among Brazilian adolescents. Our results suggest that WHtR can be a simple and powerful tool in the screening of IR in these individuals, and that the sexual maturation stage should be considered in the care protocols.
In view of these findings and considering the scarcity of publications in the literature, we hope more research will be conducted on this subject, including prospective studies that can confirm the predictive power of WHtR for IR along the sexual maturation process.
Acknowledgements
We gratefully acknowledge all of the adolescents, their parents, schools and members participating in the ERICA Study.
None.
P. R. M. L.: Conceptualisation, formal analysis, methodology, validation, investigation, visualisation, writing – original draft, writing – review and editing; A. da C. P. de A. N.: formal analysis, methodology, visualisation, writing – original draft, writing – review and editing; R. P. de T. V.: conceptualisation, formal analysis, methodology, validation, investigation, supervision, visualisation, writing – original draft, writing – review and editing.
There are no conflicts of interest.










