Globally, the age demographic structure is changing with a rapid rise in the ageing population. Presently, 0·9 billion people in the world are 60 years or older and this population is projected to rise to 2·1 billion by 2050(1). In the UK, there is an increase in the older population with 18 % aged 65 years and over and 2·4 % aged 85 years and over. Malnutrition (in this review, we only refer to undernutrition) is a common clinical and public health challenge, particularly in older adults that results in accelerated loss of independence, compromised quality of life, adverse health conditions leading to increase in hospital admissions, length of stay, as well as hospital readmission following discharge. Nearly 1·3 million people aged 65 years and over suffer from malnutrition in the UK, whilst 93 % of those affected are reported to live in the community(Reference Russell and Elia2, Reference Elia3). Malnutrition poses a major economic threat to UK healthcare, with an estimated cost of over £10 billion in England in 2011–12(Reference Elia3). Elderly malnutrition is multifactorial and is generally associated with anorexia of ageing, i.e. lack of appetite and reduced nutrient intake(Reference Soenen and Chapman4, Reference Malafarina, Uriz-Otano and Gil-Guerrero5).
Besides ageing-related physiological changes in gut and onset of early satiety(Reference Britton and McLaughlin6), such reduced nutrient intake in elderly individuals is directly or indirectly associated with progressive loss of muscle mass, decline of oral functions and coordination capabilities, all of which partly or as a whole affect the intricate process of eating(Reference Laguna, Sarkar, Chen and Watson7–Reference Laguna and Chen9). These complex physiological age-related changes are not yet fully understood but are thought to be related to lifelong accumulation of impairments at molecular, tissue and organ level. Although the process of eating is often underestimated, it involves a systematic series of well-coordinated unit operations, such as opening a package, lifting objects, cutlery manipulation, transporting the food to the mouth, closing the mouth, chewing, saliva incorporation, bolus formation and swallowing. An older adult may find difficulties in executing one or more of these important unit operations in the overall eating process that can result in reduced food intake. Indeed, there has been a vast amount of literature on dysphagia (swallowing disorder)(Reference Cichero, Steele and Duivestein10–Reference Geeganage, Beavan and Ellender12); however, focussing only on swallowing can underestimate the challenges that one might face during the entire eating process, such as transporting food to mouth.
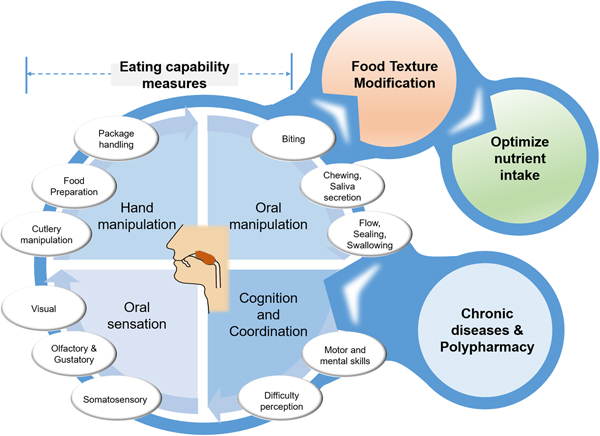
For this reason, a new term eating capability (EC) has been coined(Reference Laguna, Sarkar and Artigas8, Reference Laguna and Chen9) to collectively represent a healthy individual's endogenous capability that is directly or indirectly associated with food handling and oral management. The individual parameters needed for the eating process can probably be grouped into the following four categories: (i) hand manipulation, (ii) oral manipulation, (iii) oral sensation and (iv) cognition and coordination capabilities (Fig. 1). Under-representation of such quantitative capabilities might not be always linked to age-dependent physiological decline that has somehow been over-emphasised in the literature(Reference Rowe and Kahn13) but may be also associated with particular conditions, such as chronic diseases, multiple morbidity conditions or polypharmacy (Fig. 1). This review paper will explore the present data on EC, its individual constituents, EC score and how these capabilities are linked. We will specifically focus on hand capabilities (hand gripping force, finger force, finger touch sensitivity) and oral capabilities (bite force, tongue pressure, lip sealing pressure) with reference to the diagnostic devices (Fig. 2) that are used for their quantitative measurements. Detailed reviews on other endogenous factors, such as salivary quantity and quality(Reference Xu, Laguna and Sarkar14) and taste modification (Reference Methven, Allen and Withers15), are reported elsewhere.
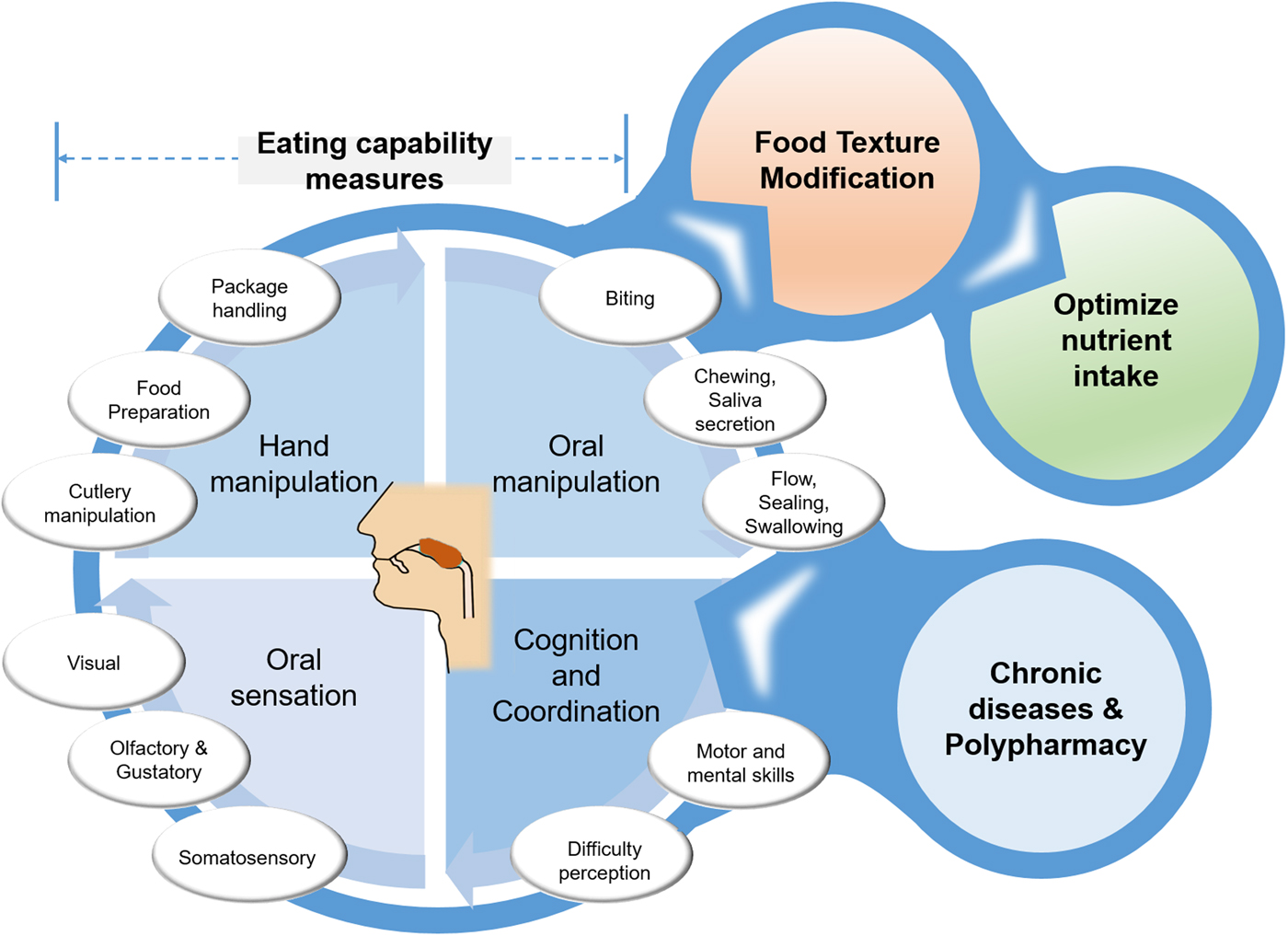
Fig. 1. (Colour online) Schematic overview of eating capability measurements that provide design inputs to food texture modifications, and may act as enabler to drive output of optimised nutrient intake. Eating capability can be negatively affected by input conditions of chronic diseases and polypharmacy.
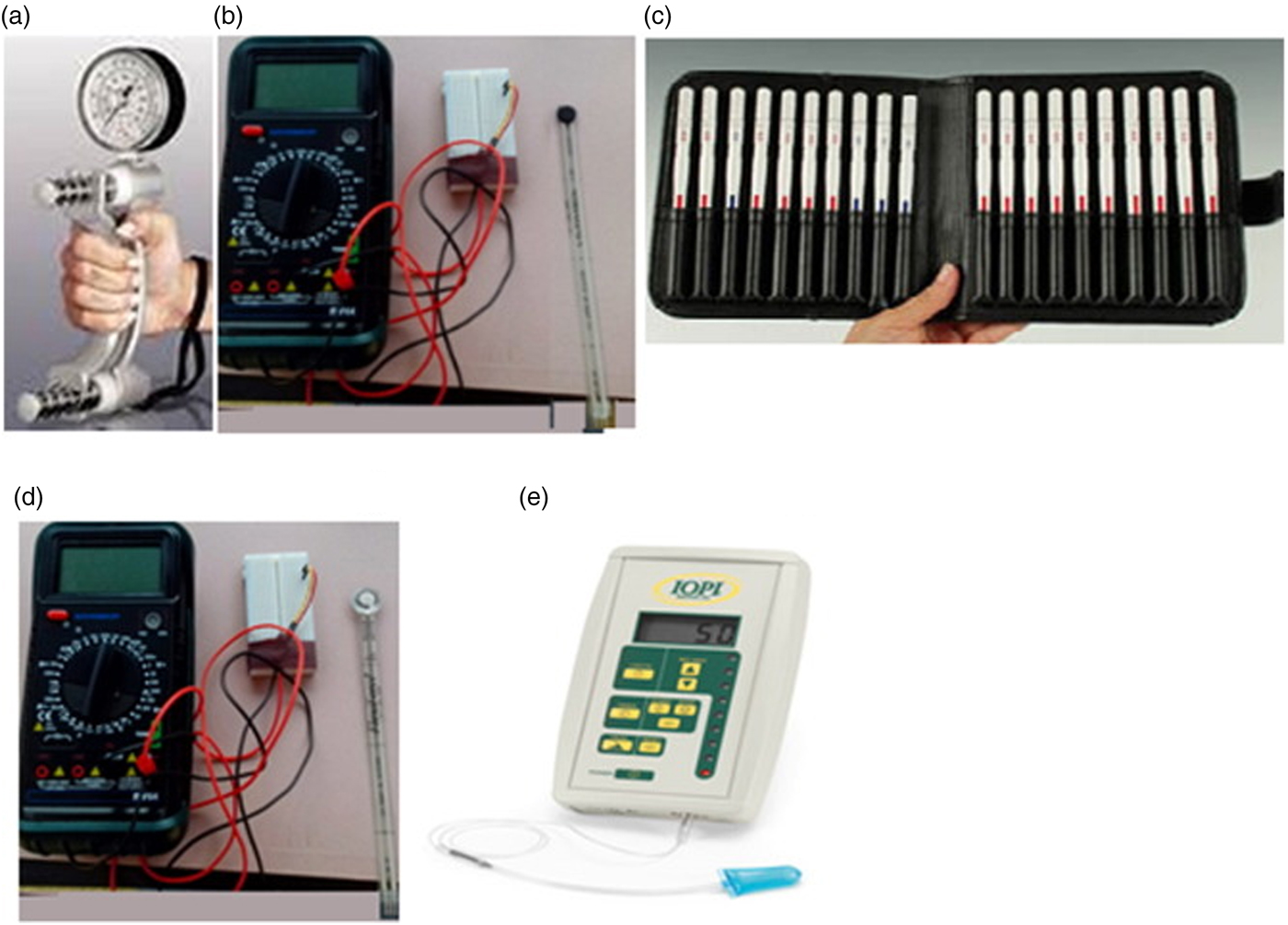
Fig. 2. (Colour online) Devices used for measuring eating capability including JAMAR dynamometer for hand gripping force (a), Flexisensor with neoprene disc for finger gripping force (b), Semmes–Weinstein monofilament for touch sensitivity (c), Flexisensor with silicone disc for bite force (d), and Iowa Oral Performance Instrument for tongue and lip sealing pressure measurements (e); Copyright© 2015 Elsevier, Reproduced with permission(Reference Laguna, Sarkar and Artigas8).
From an exogenous viewpoint, nutritionally dense foods have been traditionally offered to older adults with little emphasis on the texture and associated pleasure of eating these food items that can affect food consumption. In particular, such foods are mostly pureed and have been designed without taking into account the individual needs and abilities of older adult consumers. Hence, this review will discuss how one's EC measure can be used as objective data inputs to design personalised food with tailored textural properties that can act as an enabler to ensure safe food consumption and optimise food intake by an individual elderly consumer (Fig. 1). In this review, we will especially emphasise two key research works(Reference Laguna, Sarkar and Artigas8, Reference Laguna, Hetherington and Chen16) carried out in our laboratory with elderly individuals aged 65 years and older within the frame of EU FP7 Project OPTIFEL (2014–17). This is because these were the first two experimental works that have formalised EC for older adults, calculated EC scores to cluster older adults into capability-based archetypes and have shown promising results for the prediction of eating difficulty perception and real oral processing behaviour.
Age-related change in measures of eating capability
Hand gripping force, finger force and finger tactile threshold
For hand capability measurements, the hand gripping force is an important parameter that is a reliable indicator of upper limb function, general muscle strength and health status. Hand gripping force has been frequently used as a diagnostic parameter in clinical studies(Reference Sasaki, Kasagi and Yamada17, Reference Roberts, Denison and Martin18). This objective measure can give useful information about an individual's ability to do a range of unit operations effectively in the eating process, such as holding a coffee mug, opening a jam jar, grasping an apple to lift it and transport it from the plate to mouth. Hand gripping force is measured using an adjustable handheld dynamometer (Fig. 2(a))(Reference Laguna and Chen9) that is squeezed by the older adults with maximum effort for a few seconds with elbow flexed at 90 degrees and forearm, wrist in relaxed position. Bohannon et al.(Reference Bohannon, Peolsson and Massy-Westropp19) presented a multinational meta-analysis of the normative values for hand grip strength obtained with this dynamometer from twelve studies (3317 subjects) and concluded that age group, sex, tested side (left or right hand) affected the hand gripping force. The mean right-hand values for people aged 65 years and older were 28–41·7 and 18–24·2 kg for males and females, respectively, as compared with younger adults aged 20–40 years (53·3–54·1 and 30·6–33·2 kg for males and females, respectively). In a recent study conducted on EC(Reference Laguna, Sarkar and Artigas8), we measured right-hand gripping force in healthy British and Spanish older adults (203 subjects) and demonstrated that although age had an influence on reduction of hand gripping strength, the decline was prominent only in participants above 80 years (Fig. 3(a)). Interestingly, these values were in line with normative data for the functional grip strength of elderly population in a Singapore population (233 subjects) measured using a custom-made hand strength measurement device.

Fig. 3. Age dependency of eating capability parameters, showing right-hand gripping force (a), finger force (b), finger tactile sensitivity (c), bite force (d), tongue pressure (e) and lip sealing pressure (f) as a function of age in older adults from the UK (n 103; black bars) and Spain (n 100; white bars); Copyright© 2015 Elsevier, Data used with permission(Reference Laguna, Sarkar and Artigas8).
Finger force is an equally important parameter as decline in finger dexterity might impact one's ability to perform the eating process effectively, such as, cutlery manipulation, pulling the lid of a packaged yoghurt or ready meal's foil lid, holding a cracker or a biscuit to transport it to mouth. We measured finger gripping force in older adults(Reference Laguna, Sarkar and Artigas8) using a thin flexible force transducer connected to a multimetre with neoprene disc (Fig. 2(a)), the latter was squeezed by the elderly subjects with their thumb and index finger to record resistance. This resistance is converted into finger grip force using appropriate calibration. Based on results from 203 elderly subjects(Reference Laguna, Sarkar and Artigas8), it can be observed that finger force decreased with age (Fig. 3(a)); however, the relationship was not definitive. In fact, this result contradicts previous literature(Reference Cole20), where it has been proposed that elderly subjects generally produce more finger grip force in excess of the slip force (the margin of safety needed to prevent slipping of an object) to compensate for the reduced friction and tactile sensitivity. Besides evaluating finger grip force, researchers have emphasised the importance of tangential lift, i.e. load force to the grip surface(Reference Goodwin, Jenmalm and Johansson21) as well as tangential torques(Reference Kinoshita, Bäckström and Flanagan22). The finger grip-to-load force balance has been proposed to be automatically adjusted to a given finger-surface frictional condition. In other words, finger grip-to-load force balance is known to be largely associated with age-related changes in the surface properties of skin(Reference Diermayr, McIsaac and Gordon23). As ageing progresses, the skin becomes drier with reduction in skin hydration of the outermost layer that may in turn reduce the friction at the contact surfaces between the object and the finger. Thus, an elderly person might exert more finger grip force to hold the object to compensate for the decline in the friction force. It is noteworthy that the friction coefficient is not an intrinsic property of the skin but is highly dependent on the material chemistry and microgeometry of the surfaces, such as plastic, metals, glass, fabric with which the skin is in contact(Reference Derler, Schrade and Gerhardt24). For instance, the average friction coefficients can be low and range from 0·27 to 0·7 when skin comes in contact with textile materials(Reference Derler, Schrade and Gerhardt24). Conversely, considerably high friction coefficients of skin can be encountered against dry, smooth glass (2·18 (sd 1·09); range: 0·39–5) whereas lower coefficients on wet smooth glass (0·61 (sd 0·37); range: 0·07–2·12). Hence, it is important not only to understand the finger grip force but also to examine the friction force against a variety of surfaces which an older adult may encounter.
Finger tactile sensitivity is crucial for identifying food texture and can lead to food rejection. Older adults often suffer from marked degradation in tactile sensitivity as a function of normal ageing process that can result in slipping objects. In other words, the mechanoreceptor tactile thresholds may increase with ageing. Finger tactile sensitivity measurements are generally measured using Semmes–Weinstein monofilament test (Fig. 2(c)). The monofilament of different forces is pressed in perpendicular direction against the surface by elderly participants and the first monofilament that is detected by the participant is recorded as the tactile threshold. Thornbury et al.(Reference Thornbury and Mistretta25) suggested that touch sensitivity decreases, i.e. the threshold increases with age significantly. However, in touch sensitivity trial conducted in our laboratory(Reference Laguna, Sarkar and Artigas8), most of the elderly individuals had a relatively low threshold and most participants were able to detect < 0·16 g force (Fig. 3(c)). Tactile sensitivity did not correlate with age or sex, but was largely associated with some health conditions, such as arthritis, Parkinson's disease.
Bite force, tongue pressure and lip sealing pressure
Optimum oro-facial muscle force involving lips, tongue and teeth are of central importance to a normal eating process. Once the food is consumed, it is accommodated inside the mouth with lip closure, chewed by exerting appropriate bite force to experience the texture of the product and subsequently reduce the structural size of the ingested food, dilution and lubrication by saliva, compression between the tongue and oral palate by a range of tongue forces and other orofacial muscular forces followed by swallowing of the bolus(Reference Sarkar, Ye and Singh26–Reference Laguna and Sarkar28). Consequently, any decline in oro-facial muscular capability can directly affect one's eating process and in turn reduce food intake.
Maximum bite force is used as a capability measure, which can directly influence fragmentation of food, chewing and mastication. To ensure safe food mastication, one's bite force should be higher than the yield force require to fragment a food material. For instance, a food with a yield force of 100 N may be sensed as soft by a person who can exert 300 N force, but will be perceived as hard and almost not friable to the one who could only apply a maximum of 110 N(Reference Chen29). In general, bite force is measured using a flexible transducer (Fig. 2(d)) that is placed between a pair of teeth(Reference Koc, Dogan and Bek30), similar to the sensor used for finger force measurement. We demonstrated that bite force decreased with age (Fig. 3(d)); however, influence of preserving natural teeth was the deterministic factor for higher bite force as compared with that of the age(Reference Laguna, Sarkar and Artigas8). This is in line with previous studies, where bite force was significantly smaller among the denture wearers than among the dentate persons(Reference Helkimo, Carlsson and Helkimo31). In other words, the greater number of natural teeth, greater is the bite force and ease of food mastication. Another study conducted with 850 independently living people over the age of 60 years, also postulated that tooth loss is not a consequence of physiological ageing but pathological ageing, and thus, reduction of bite force cannot be considered as a natural effect of ageing(Reference Ikebe, Nokubi and Morii32).
During the process of swallowing, the tongue positions the food bolus and plays a critical role in the propulsion of the bolus with the help of tongue pressure arising from its contact against the hard palate(Reference Stone and Shawker33, Reference Ono, Hori and Nokubi34). Obviously, optimal swallowing performance requires the complex movements of the tongue to transport the bolus safely and efficiently. Maximum isometric tongue pressure can be measured using a simple clinical device with a disposable tongue bulb (Fig. 2(e)) that can be placed in the mouth between the tongue and the palate, which is linked to a pressure transducer recording the maximum tongue–palate isometric pressure. Unlike other oro-facial muscle forces, tongue pressure parameter has emerged as a measure of considerable clinical and research interest in the field of dysphagia over the past two decades(Reference Steele35). Lip closure is another crucial oral function that helps to retain the food or beverages inside the mouth. This is even more critical during swallowing when the pressure is elevated within the oral cavity(Reference Laguna and Chen9). The lip sealing capability can be measured by quantifying the magnitude of maximal closing force that is held between the upper and lower lips using the same sensor that is used to measure tongue pressure (Fig. 2(e)). Both tongue and lip sealing pressure showed no correlation with age increment in the study conducted with 203 elderly participants (Figs. 3 (e) and (f))(Reference Laguna, Sarkar and Artigas8). A recent study with 201 older adults aged ≥65 years demonstrated that magnitude of tongue and lip pressure were inversely correlated with food intake(Reference Sakai, Nakayama and Tohara36). The same group of authors conducted a cross-sectional study(Reference Sakai, Nakayama and Tohara37) with 174 older adults aged 65 years and older in rehabilitation and demonstrated that isometric tongue strength was associated with nutritional status assessed (β-coefficient = 0·74, 95 % CI 0·12, 1·35, P = 0·019) using mini nutritional assessment. It is worth pointing out that tongue plays a crucial role in controlling the flow of food–saliva mixture (bolus) and fragments, within the oral cavity as well as swallowing. Tongue plays a series of well-coordinated roles in mastication and swallowing by controlling the pressure against the hard palate(Reference Hori, Ono and Nokubi38). Decreased tongue strength and consequently tongue pressure exerted against the oral palate can result in limited or abnormal transportation of the food bolus to the oesophagus, which can lead to aspiration, oral residues, longer meal times and finally low food consumption(Reference Sakai, Nakayama and Tohara37). Overall, this suggests that objective EC measures can be used not only to understand health status but also to get indications about nutrient intake. Also, focussing on objective EC measure rather than age might help to design personalised food for elderly; however, the research evidences in this area is at its infancy and expected to grow considering the rapid rise in ageing population and associated malnutrition challenges.
Relationship between hand and oro-facial muscle forces
Although oro-facial muscle force measures can be directly useful to understand effectiveness to perform oral functions and eating process, a major issue is that many of these devices are not easy to use in care homes. Hence, studies have been attempted to understand whether hand grip strength can be used as an indirect measure for oral functions. For example, Sakai et al.(Reference Sakai, Nakayama and Tohara37) conducted a multivariate linear regression analysis and revealed that isometric tongue strength was correlated with grip strength (β-coefficient = 0·33, 95 % CI 0·12, 0·54, P = 0·002) in older adults. Similarly research work in our laboratory(Reference Laguna, Sarkar and Artigas8) also demonstrated strong linear relationships between hand gripping strength and most of the oro-facial muscle forces (bite force, tongue pressure, lip sealing pressure) in the UK (Fig. 4(a)) and Spanish (Fig. 4(b)) subjects (except for lip sealing pressure, where it was a polynomial relationship in the latter). A related study was conducted in 381 persons older adults aged 67–74 years to understand the relationship between hand grip strength and self-reported chewing ability(Reference Moriya, Tei and Yamazaki39). The masticatory ability was classified into three groups: (1) ability to chew all kinds of food, (2) slightly hard food and (3) only soft or pureed foods. As can be expected, hand grip strength was significantly lower in those individuals who could chew only soft or pureed food than in those individuals who could chew all kinds of food inferring that chewing ability was significantly related to hand grip strength after adjusting for the skeletal muscle mass, dentition status and background factors. In another study in Japan(Reference Eto and Miyauchi40) with independent 159 community-dweller elderly aged 65 years old and above showed that maximum occlusal force was significantly correlated with the hand grip strength (r = 0·382, P < 0·01). All these observed relationships between hand grip and oro-facial muscle strengths indicate possibilities of using hand gripping force by the carers as a non-invasive parameter to predict oral functions.
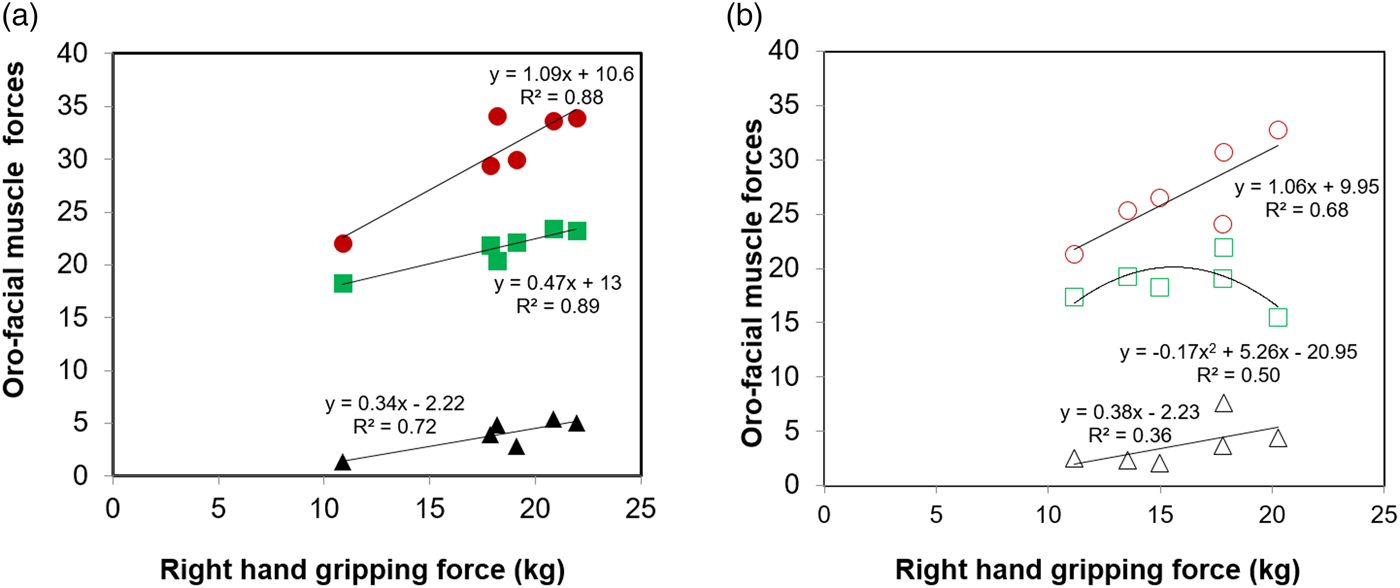
Fig. 4. (Colour online) Relation of right-hand gripping force with oro-facial muscle forces (maximum bite force (▲), maximum tongue pressure (●) and lip sealing pressure (■) of older adults in the UK (closed symbols)); (a) and Spain (open symbols) (b), respectively. Each data point represents the mean data of forces from participants in each of the age classes (years old), i.e. 65–70, 70–75, 75–80, 80–85, 85–90 and 90+. Black lines show linear-regression best fits to the observed values except for lip sealing pressure relationship in Spain, latter shows a polynomial-fit. Copyright© 2015 Elsevier, Data used with permission(Reference Laguna, Sarkar and Artigas8) for (a) and Copyright© 2015 Cambridge University Press, reproduced with permission(Reference Laguna, Sarkar and Chen46) for (b).
Eating capability score
The literature on elderly population has mostly examined a defined older group and compared their behaviour with younger groups. However, it must be recognised that elderly population of 65 years and older are not homogenous in their needs, expectations, capabilities and frailty within the same age group. For example, a recent European survey (Finland, France, Poland, Spain and UK) was conducted with over 400 elderly people aged 65 years and older and they were categorised into three groups based on their different levels of dependency (category 1: participants living at home needing help for food purchasing; category 2: participants living at home needing help for meal preparation or meal delivery; category 3: participants living in nursing homes/sheltered accommodation)(Reference Mingioni, Mehinagic and Laguna41, Reference Laguna, Mingioni and Maitre42). Laguna et al.(Reference Laguna, Mingioni and Maitre42) suggested that category 1 participants did not perceive difficulties during meal preparation and reported some level of difficulties in hand manipulation and oral processing (<30 %), whereas about 60 % of older adults in categories 2 and 3 suffered from such eating difficulties. Besides these self-reported studies, structured protocols of observation of meals have been used to detect eating difficulty(Reference Medin, Windahl and von Arbin43, Reference Jacobsson, Axelsson and Österlind44). For instance, Jacobsson et al.(Reference Jacobsson, Axelsson and Österlind44) used observational experiments together with video-recording and interviews during meal consumption in a small group of adults aged 70 years and older to understand eating difficulties. Authors reported not only swallowing-related difficulties but also other issues in terms of preparing and transporting the food to the mouth. As one might recognise, assessment of capability of an individual has been largely based on subjective measurements traditionally.
Hence, a composite score termed as EC score was developed(Reference Laguna, Sarkar, Chen and Watson7, Reference Laguna, Sarkar and Artigas8, Reference Laguna, Hetherington and Chen16) that can serve as a reliable multifactorial objective score to categorise elderly populations into different groups based on their individual abilities rather than age. To do the same, five measurable parameters, i.e. right-hand gripping force, right-hand finger gripping force, finger tactile threshold, bite force, tongue pressure, were selected to calculate a composite EC score (equation 1)(Reference Laguna, Sarkar and Artigas8), where each of these parameters was normalised v. the strength of the strongest participant within that measured parameter:
 $$\eqalign{{\rm EC\;\ score} & = \left( {\displaystyle{{{\rm R}{\rm H}_{{\rm Par}}} \over {{\rm R}{\rm H}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm R}{\rm F}_{{\rm Par}}} \over {{\rm R}{\rm F}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm T}{\rm S}_{{\rm Par}}} \over {{\rm T}{\rm S}_{{\rm Str\; Par}}}}} \right) \cr & \ {\rm +} \left( {\displaystyle{{{\rm B}{\rm F}_{{\rm Par}}} \over {{\rm B}{\rm F}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm T}{\rm P}_{{\rm Par}}} \over {{\rm T}{\rm P}_{{\rm Str\; Par}}}}} \right),}$$
$$\eqalign{{\rm EC\;\ score} & = \left( {\displaystyle{{{\rm R}{\rm H}_{{\rm Par}}} \over {{\rm R}{\rm H}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm R}{\rm F}_{{\rm Par}}} \over {{\rm R}{\rm F}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm T}{\rm S}_{{\rm Par}}} \over {{\rm T}{\rm S}_{{\rm Str\; Par}}}}} \right) \cr & \ {\rm +} \left( {\displaystyle{{{\rm B}{\rm F}_{{\rm Par}}} \over {{\rm B}{\rm F}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm T}{\rm P}_{{\rm Par}}} \over {{\rm T}{\rm P}_{{\rm Str\; Par}}}}} \right),}$$where RH is the right-hand gripping force (kg), RF is the right-hand finger gripping force (kg), TS is the tactile sensitivity threshold (g), BF is the bite force (kg), TP is the tongue pressure (kPa), subscripts Par and Str Par represent the individual and strongest individual scoring the highest in that particular test, respectively. The EC score was used to characterise participants from weakest to strongest in groups, i.e. participants with EC≤2 were placed in group 1 (the weakest group); participants with EC>2 and ≤4 were placed in group 2, and so on(Reference Laguna, Sarkar and Artigas8).
The EC score was further updated using equation (2) considering the importance of coordination capability(Reference Laguna, Sarkar, Chen and Watson7, Reference Laguna, Hetherington and Chen16), importance of both right- and left-hand forces rather than just right-hand force as used in equation (1) and removing the less reproducible parameters from equation (1), such as finger force and tactile sensation:
 $$\eqalign{{\rm EC\;\ score} & = \displaystyle{{\left( {\displaystyle{{{\rm R}{\rm H}_{{\rm Par}}} \over {{\rm R}{\rm H}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm L}{\rm H}_{{\rm Par}}} \over {{\rm L}{\rm H}_{{\rm Str\; Par}}}}} \right)} \over 2} \cr & + \left( {\displaystyle{{{\rm B}{\rm F}_{{\rm Par}}} \over {{\rm B}{\rm F}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm T}{\rm P}_{{\rm Par}}} \over {{\rm T}{\rm P}_{{\rm Str\; Par}}}}} \right) \cr & + \displaystyle{{\left( {\displaystyle{{{\rm RD}} \over {{\rm R}{\rm D}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm L}{\rm D}_{{\rm Par}}} \over {{\rm L}{\rm D}_{{\rm Str\; Par}}}}} \right)} \over 2},}$$
$$\eqalign{{\rm EC\;\ score} & = \displaystyle{{\left( {\displaystyle{{{\rm R}{\rm H}_{{\rm Par}}} \over {{\rm R}{\rm H}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm L}{\rm H}_{{\rm Par}}} \over {{\rm L}{\rm H}_{{\rm Str\; Par}}}}} \right)} \over 2} \cr & + \left( {\displaystyle{{{\rm B}{\rm F}_{{\rm Par}}} \over {{\rm B}{\rm F}_{{\rm Str\; Par}}}}} \right) + \left( {\displaystyle{{{\rm T}{\rm P}_{{\rm Par}}} \over {{\rm T}{\rm P}_{{\rm Str\; Par}}}}} \right) \cr & + \displaystyle{{\left( {\displaystyle{{{\rm RD}} \over {{\rm R}{\rm D}_{{\rm Str\; Par}}}}} \right){\rm +} \left( {\displaystyle{{{\rm L}{\rm D}_{{\rm Par}}} \over {{\rm L}{\rm D}_{{\rm Str\; Par}}}}} \right)} \over 2},}$$where LH is the left-hand gripping force (kg), RD is the right-hand dexterity count and LD is the left-hand dexterity count (using manual dexterity kit). The role of EC score on difficulty perception and oral processing of real food and gels is discussed in the next section.
Eating capability score as predictor of eating difficulty perception/real-life oral processing behaviour
To understand the application of EC, tests(Reference Laguna, Barrowclough and Chen45) were conducted with eleven young subjects with a range of food with different textural complexity to understand if individual physical forces (hand or oral forces) were important to understand food difficulty perception. Interestingly, no relationship could be established between individual's dominant hand grip force, isometric tongue pressure, bite force and food perception difficulty for the young participants. This was attributed to the selected young population having significantly higher hand force:tongue force ratio, which might not interfere with their eating process. It appeared obvious that EC measurement might be more useful for the elderly population, where one or more capability parameters can be limiting. To understand the relevance of EC score for elderly participants, Laguna et al.(Reference Laguna, Sarkar and Artigas8, Reference Laguna, Sarkar and Chen46) grouped British and Spanish participants into four independent groups based on EC score using equation (1) and older adults rated food images on how difficult they perceived them to be manipulated by hand (e.g. cutlery manipulation, cutting or lifting the food) or in mouth (e.g. chewing, biting, swallowing). It was demonstrated that participants from the weakest EC groups having composite EC score <6·64 perceived fibrous and hard food products significantly more difficult to eat than participants with higher EC score (9·95). This strongly suggested that EC score can be an input feature for personalised food product design for the elderly population.
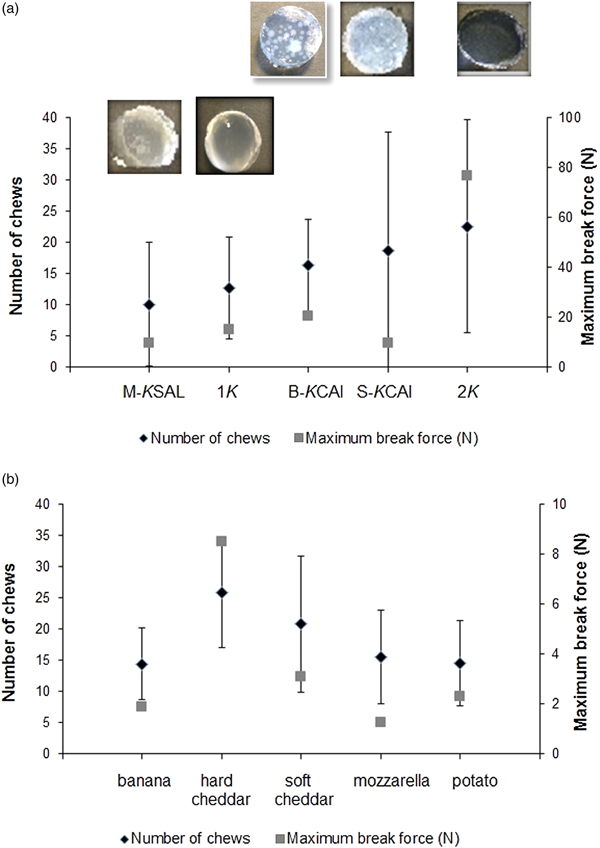
To validate whether EC concept holds promise for predicting eating difficulty in real-life food oral processing scenarios, i.e. chewing cycles, bolus-swallowing time(Reference Laguna, Hetherington and Chen16) rather than subjective perception as studied previously(Reference Laguna, Sarkar and Artigas8), elderly subjects (n 31) were asked to eat model and real foods. These model foods were hydrogels(Reference Laguna and Sarkar47) that were designed in our laboratory with different degrees of inhomogeneity (i.e. the inclusion of different sizes of calcium alginate microgel particles to a κ-carrageenan continuous network). As can be observed in Fig. 5(a)(Reference Laguna, Hetherington and Chen16), the number of chews needed to fracture the gels did not correlate significantly with the instrumental hardness of the gels. The gels chosen were harder than the food products (Fig. 5(b)). However, when the maximum force at break was similar, the time in mouth was dependent on the food structural heterogeneity, and the time in mouth increased with the heterogeneity increment (e.g. number of beads). In this study, it was demonstrated that although EC score allowed grouping of the elderly participants, it was not suitably stratified and all the groups had relatively low EC score with the most capable group having EC score 3·23. The EC score was not sufficiently correlated to real eating difficulty perception. Interestingly, the bite force was the key discriminating parameter in distinguishing bite, oral processing time, number of chews and preference. This suggests a non-composite scoring system beyond EC score, such as an individual measure (e.g. bite force or tongue pressure) may be more important in predicting eating difficulty, however this cannot be generalised at this early stage. Also, it is worth pointing out that EC score might require further refinement to categorise elderly individuals of similar capability into independent groups that can be beneficial to develop the creation of food of just-right texture and desired oral processing properties (chew cycles, swallowing time). Besides EC, another concept termed oral comfort has also been coined(Reference Vandenberghe-Descamps, Labouré and Septier48) recently that covers multidimensional oral sensations perceived by older adults including ease of chewing, humidifying and swallowing as well oral pain sensations that might occur due to decline in oral comfort, for e.g. oral comfort may be lower for dry textures. Oral comfort has been defined using a set of factors determined from a discriminable questionnaire. Future work is needed to explore the relationship between oral health, oral comfort and EC to generate a clear brief for food design for older adults from texture viewpoints.
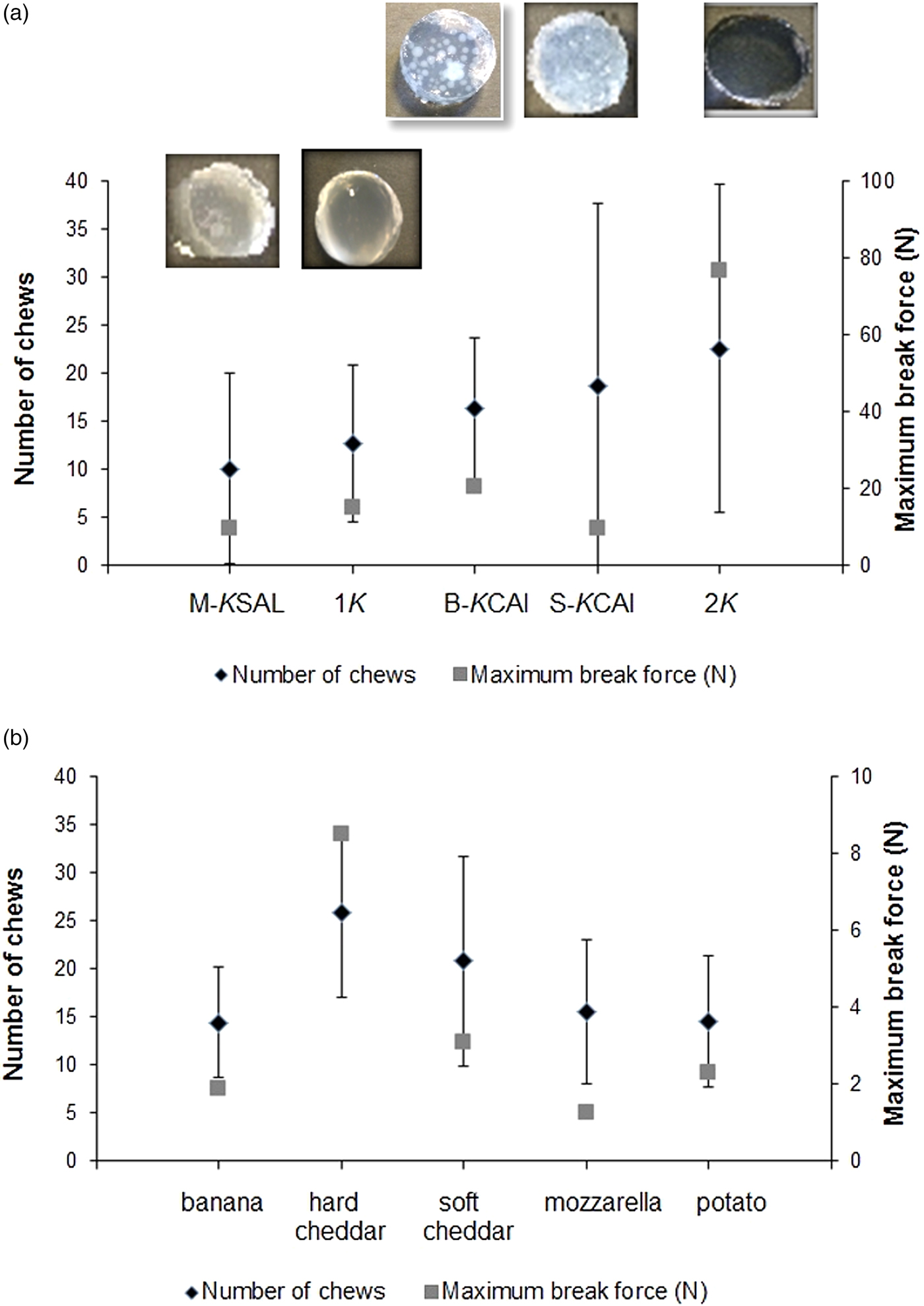
Fig. 5. Relation among samples, number of chew and maximum force at break during oral processing by older adults (n 30) for model foods (hydrogels) with controlled mechanical properties with visual corresponding images (a) and food products (b). Nomenclatures 1κ and 2κ represent hydrogels containing 1 wt % and 2 wt % κ-carrageenan, respectively, M-κSAl represents mixed hydrogel containing 2 wt % κ-carrageenan + sodium alginate, B-κCAl represents hydrogel with structural inhomogeneity containing 1 wt % κ-Carrageenan + big calcium alginate beads (mean size 1210 µm) and S-κCAl represents hydrogels with structural inhomogeneity containing 1 wt % κ-carrageenan + small calcium alginate beads of mean size 1210 µm. Copyright© 2016 Elsevier. Used with permission(Reference Laguna, Hetherington and Chen16, Reference Laguna and Sarkar47).
Future perspectives on food texture modification
In general, texture-modified foods designed for the elderly are the foods that are softened by processing, such as pureed as well as liquids that have been modified in viscosity to various extents by physical or chemical means(49). Since the rationale behind such softer food development is to address dysphagia patients, the main textural parameters used for such texture-modified food design includes hardness (hard to soft) and cohesiveness (ability of food particles to stick to each other to form a swallowable bolus)(Reference Aguilera and Park50). However, it has been elucidated that not only endogenous factors such as bite force, and exogenous factors such as consistency (hardness) of food but also the heterogeneity of the matrix affect food oral processing behaviour (number of chews and time in mouth)(Reference Laguna, Sarkar and Artigas8). This suggests that optimised food design for the elderly should not only focus on just-right texture but also attempt just-right structural heterogeneity that can act as an enabler to increase food intake in people with low EC score or reduced individual EC measures (Fig. 1).
Besides modifying viscosity by using thickeners, one of the strategies that can be used to create pleasurable texture can be to use microgel particles as discussed previously with calcium alginate particles. Such soft microgel particles made up of alginate, whey protein or starch with or without oil can be used not only to have an impact on increasing viscosity but also to modify the lubrication aspects of the food(Reference Laguna and Sarkar47, Reference Torres, Andablo-Reyes and Murray51–Reference Sarkar, Kanti and Gulotta55) for older adults, who generally suffer from dry mouth conditions due to lack of secretion of bio-lubricant saliva(Reference Xu, Laguna and Sarkar14, Reference Laguna and Sarkar28). Besides textural properties, such microgel particles can be used to modify food structural complexity and also to encapsulate and deliver essential fat-soluble vitamins, such as vitamin D, which is much needed for older adults suffering from vitamin D deficiency or insufficiency(Reference Boucher56).
It is well known that pureed foods are often associated with a decreased food intake due to the unpleasant changes in appearance, texture and mouthfeel and thus may result in increased incidences of malnutrition(Reference Keller, Chambers and Niezgoda57). Hence, there has been increased research efforts to convert pureed foods into three-dimensional (3D) forms via appropriate viscosity enhancement and moulding so that the food resembles its natural shape. Studies(Reference Farrer, Olsen and Mousley58, Reference Cassens and Johnson59) have demonstrated that using a moulded smooth puree diet can increase nutrient intake as compared with the non-moulded pureed version in a nursing home setting. Recently, texture modification has achieved attention due to recent advancements in sophisticated technologies, such as 3D printing and food-grade printable materials for innovative food textural design(Reference Lipton, Cutler and Nigl60, Reference Dankar, Haddarah and Omar61). In particular, scientists in EU Project PERFORMANCE have developed customised nutrition of the elderly using 3D printed food, where the pureed food is endowed with ‘the best clone possible’, i.e. transformed into its original shape via jet printing technology, providing the same texture and appearance, with added health benefits. Although this is still in the early stages of development, 3D printing can be immensely useful to design precision foods with just-right texture and just-right structure created with optimised nutrient levels for elderly population with known EC.
In summary, EC is a relatively recent undertaking in elderly food management. There are still large gaps in knowledge related to ageing and EC and the role these may play in predicting oral functions and ultimately oral comfort and eating difficulty. As our ageing population increases, more research studies may help us to better understand those capabilities, irrespective of age groups within the elderly population. Ultimately, such data inputs from EC measures should be used to drive objective food texture modifications using sophisticated technologies, such as 3D printing in order to design personalised food and optimise food intake rather than designing ‘blanket’ unplesurable pureed food for the entire elderly population.
Acknowledgements
I thank the Nutrition Society for the invitation to give the presentation at the Summer Conference 2018 in Leeds UK, and the opportunity to prepare the present paper. I gratefully acknowledge the contribution of Dr Laura Laguna (currently at the Institute of Agrochemistry and Food Technology (IATA, CSIC), Valencia, Spain), who collected and analysed all the data in the eating capability studies of elderly subjects within the EU FP7 project OPTIFEL. Finally, I would sincerely thank Dr Mel Holmes from the University of Leeds, Leeds, UK for his proof-reading and editorial corrections.
Financial Support
This work was supported by the European Union's Seventh Framework Programme for research, technological development and demonstration under Grant Agreement No. Kbbe-311754 (OPTIFEL).
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of literature search and preparation of the paper.