Vitamin D status is best assessed by serum 25-hydroxyvitamin D (S-25(OH)D) concentration(Reference Ross, Taylor and Yaktine1). The risk of vitamin D insufficiency or inadequacy, measured as S-25(OH)D <50 nmol/l(Reference Ross, Taylor and Yaktine1–3), is a public health issue among indigenous populations living at Northern latitudes, but of greater concern is the situation among immigrants living in the region(Reference Van Der Meer, Middelkoop and Boeke4–Reference Lips and de Jongh6). Besides the major protective role of adequate vitamin D status in skeletal health(Reference Ross, Taylor and Yaktine1,2) , there is concern about other health conditions potentially associated with low vitamin D status (e.g. cancers, cardiovascular diseases and diabetes)(Reference Bouillon, Marcocci and Carmeliet7,Reference Rejnmark, Bislev and Cashman8) especially among groups most vulnerable to vitamin D deficiency (S-25OHD <30 nmol/l). Negative associations between low 25(OH)D concentrations and non-skeletal outcomes have been reported in observational studies(Reference Bouillon, Marcocci and Carmeliet7,Reference Rejnmark, Bislev and Cashman8) . The benefits of vitamin D supplementation in non-skeletal conditions were observed on depression, blood pressure, respiratory tract infections and all-cause mortality in some meta-analyses(Reference Rejnmark, Bislev and Cashman8). Nevertheless, findings from observational studies were mostly not supported by randomised controlled trials, meta-analyses and systematic reviews(Reference Rejnmark, Bislev and Cashman8).
Studies have shown poor vitamin D status among non-Western immigrants living in European countries compared with native European populations(Reference Van Der Meer, Middelkoop and Boeke4,Reference Spiro and Buttriss5) . In Nordic countries, insufficient vitamin D status has been reported among immigrants compared with their host population(Reference Andersson, Björk and Kristiansson9–Reference Wändell11). Likewise, in Finland, higher rates of vitamin D deficiency and insufficiency have previously been reported among immigrant women (Somali and Bangladesh) than in native Finnish women(Reference Islam, Viljakainen and Kärkkäinen12). Disparity in vitamin D status between Caucasian Finnish women and immigrant women of Somali background was also confirmed recently as the screening results of our study revealed a higher prevalence of vitamin D insufficiency status among Somalis (56 %) compared with Finns (9 %)(Reference Adebayo, Itkonen and Öhman13).
Besides an endogenous production of vitamin D3 through the action of UVB radiation on the skin, dietary and/or supplemental intake are the other established sources of vitamin D as well as determinants of vitamin D status(Reference Ross, Taylor and Yaktine1,Reference Van Der Meer, Middelkoop and Boeke4,Reference Lamberg-Allardt, Brustad and Meyer14) . In addition, several sociodemographic and lifestyle variables, such as African race, dark skin colour, low exposure to sunlight, wearing concealing clothes, higher BMI, physical inactivity, smoking and low education status, have been reported as determinants of low vitamin D status(Reference Bouillon, Marcocci and Carmeliet7,Reference Granlund, Ramnemark and Andersson15–Reference Jääskeläinen, Knekt and Marniemi17) .
Recent nationally representative studies have reported adequate vitamin D status among the general Finnish population(Reference Raulio, Erlund and Männistö18,Reference Jääskeläinen, Itkonen and Lundqvist19) . As immigrants are most vulnerable to low vitamin D status, prevalence data for vitamin D deficiency and inadequacy are of great public health importance. The aim of this study was to investigate the vitamin D status in terms of S-25OHD concentration and its determinants and consumption of major dietary sources of vitamin D among three immigrant groups living in Finland: Russians, Somalis and Kurds. We also compared the vitamin D status of immigrant groups with that of the general Finnish population.
Methods
Design and study population
This study is based on the data from two different larger samples: Migrant Health and Wellbeing Study (Maamu) and Finnish Health 2011 Survey(Reference Castaneda, Rask and Koponen20,Reference Lundqvist and Mäki-Opas21) . The selection of participants is described in the flowchart of the study population (Fig. 1). Maamu was the first population-based health survey conducted among immigrants in Finland. The participants were randomly selected from the National Population Register. A total of 3000 adults aged 18–64 years of Russian, Somali or Kurdish origin (1000 persons from each immigrant group) were invited to the study through mailed letters. Maamu data were collected between 2010 and 2012 in the cities of Helsinki (60°N), Espoo (60°N), Vantaa (60°N) (2010–2011), Turku (60°N) (2011), Tampere (62°N) and Vaasa (63°N) (2012). The inclusion criteria were country of birth (Somalia, Iraq/Iran or Russia/former Soviet Union), mother tongue (Kurdish Sorani dialect and Russian/Finnish – for Kurdish and Russian participants, respectively), minimum of 1 year residency in Finland and living in one of the aforementioned six cities. Data were collected in the participants’ mother tongue by multilingual trained personnel who spoke both the language of the respective immigrant participants and Finnish. Further details on Maamu are presented elsewhere(Reference Castaneda, Rask and Koponen20,Reference Skogberg, Laatikainen and Koskinen22) .
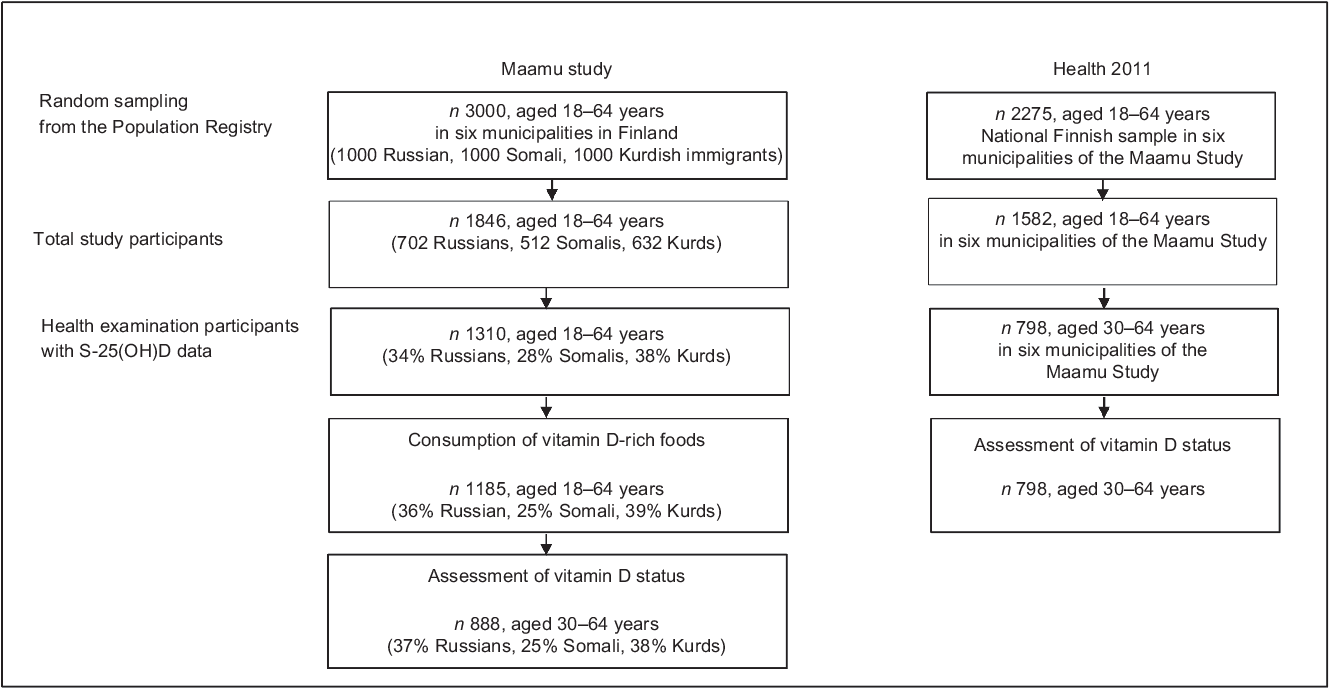
Fig. 1 Flowchart of the study population. Maamu, Migrant Health and Wellbeing Study; Health 2011, Health 2011 Survey; S-25(OH)D, serum 25-hydroxyvitamin D
The Health 2011 Survey is a national representative survey carried out among Finnish adults living in mainland Finland. The participants (n 2275, aged 18–64 years) of the Health 2011 Survey were selected to the general Finnish population reference group from the corresponding six cities where Maamu was conducted. Data of Health 2011 Survey were collected through questionnaires, interviews and a comprehensive health examination. The Health 2011 Survey was conducted in the same timeframe (2011–2012) and protocol comparable with Maamu, and both studies were conducted by the Finnish Institute for Health and Welfare (THL)(Reference Lundqvist and Mäki-Opas21,Reference Härkänen23) . S-25(OH)D data for Maamu and Health 2011 Survey in their entirety were previously presented(Reference Jääskeläinen, Itkonen and Lundqvist19,Reference Cashman, Dowling and Škrabáková24) , but not stratified by ethnic groups and with different objectives from that of this current paper. Moreover, Maamu data were earlier studied in terms of consumption of fish and some other foods, but not in relation to vitamin D(Reference Adebayo, Itkonen and Koponen25).
Current study sample
Data from 45 % of the invited Russian- (n 446), 36 % of Somali- (n 364) and 50 % of Kurdish-origin (n 500) persons aged 18–64 years who participated in Maamu, and 798 Finnish adults aged 30–64 years in the nationally representative Health 2011 Survey as a reference group, were used in this study. Included subjects were those with available data on S-25(OH)D concentration. Data from 328 Russian, 221 Somali and 339 Kurdish participants aged 30–64 years were compared with the Finnish reference group. This was due to limited available data on S-25(OH)D concentration among the younger Finnish reference group (aged 18–29 years). The studied groups are subsequently referred to as Russians, Somalis, Kurds (from Maamu) and Finns (from Health 2011 Survey).
Participants’ characteristics
In both Maamu and Health 2011 Survey, information on age, sex and country of origin (for immigrants) was obtained from the sampling frame. Data on education and smoking status were collected through interviews in Maamu and Health 2011 Survey. Information about alcohol consumption and physical activity was obtained through interviews in Maamu and through a self-administered health questionnaire in Health 2011 Survey. Data about the use of vitamin D supplements were obtained during interviews in Maamu and as a part of the FFQ in Health 2011 Survey. The questions used to assess educational status and lifestyle factors (smoking, alcohol consumption, physical activity and use of vitamin D supplements) in both studies were similar and allowed comparison. Educational status was assessed based on the questions about the participants’ educational attainment; this includes education both in home countries and in Finland for immigrants. Responses to the questions were dichotomised into having less than high school and high school education or more. Smoking status was categorised as daily smoking, and options to the questions were dichotomised into ‘no’ and ‘yes’. Alcohol consumption was rated as either excess consumption (yes) or not (no) based on the Alcohol Use Disorders Identification Test (AUDIT-C) scores for men and women with cut-offs ‘no’ = AUDIT <6 points for men and <5 points for women; ‘yes’ = AUDIT ≥6 points for men and ≥5 points for women(Reference Aalto, Tuunanen and Sillanaukee26,Reference Tuunanen, Aalto and Seppä27) . Physical activity was assessed by the type and intensity of the activities engaged in by the participants. The response was categorised into inactive/less active and highly active. With regard to the use of vitamin D supplements, the participants were asked whether they had used supplements (single-ingredient vitamin D supplements, or multivitamins, or with minerals) during the last 12 months. The response to the question was ‘no’ or ‘yes’. BMI was calculated based on height and weight (i.e. weight (kg)/height (m2)) measured during health examination. It was dichotomised into two categories: non-obese (<30 kg/m2) and obese (≥30 kg/m2)(28).
Measurement of S-25(OH)D
As part of health examination, fasting blood samples were collected in both studies. The minimum average fasting period was 8 h for the four study groups(Reference Skogberg, Laatikainen and Koskinen22). After serum separation, samples were stored frozen at –70°C until analysis(Reference Jääskeläinen, Itkonen and Lundqvist19,Reference Skogberg, Laatikainen and Koskinen22) . In Maamu, blood sampling was performed from December 2010 to April 2012 and from August to December 2011 in Health 2011 Survey. S-25(OH)D concentrations in both studies were originally analysed by chemiluminescent immunoassay (Architect ci8200; Abbott Laboratories) in the laboratory of the Finnish Institute for Health and Welfare. Total S-25(OH)D concentrations from both studies were standardised according to the Vitamin D Standardisation Program (VDSP) by means of a certified LC-MS/MS method at the University College Cork, Ireland(Reference Jääskeläinen, Itkonen and Lundqvist19,Reference Cashman, Dowling and Škrabáková24) . Interassay CV for the LC-MS/MS analysis of S-25(OH)D was <5 % in both Maamu and Health 2011 Survey. The LC-MS/MS method was certified under the Centers for Disease Control and Prevention Vitamin D Standardisation Certification Program (https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures-508.pdf). VDSP ensures measurement accuracy and more valid comparison of S-25(OH)D data across studies and time(Reference Sempos, Vesper and Phinney29). The VDSP protocol has been described in detail elsewhere(Reference Cashman, Dowling and Škrabáková24,Reference Binkley and Sempos30) . In the present study, we categorised season of blood sampling as summer (April–September) and winter (October–March). In line with the Institute of Medicine (IOM)-suggested S-25(OH)D thresholds, which have been adopted in both the Nordic and Finnish nutrition recommendations, the present study used the following definitions of vitamin D status: deficient (S-25(OH)D <30 nmol/l); insufficient (S-25(OH)D = 30–49·9 nmol/l); and sufficient (S-25(OH)D ≥50 nmol/l)(Reference Ross, Taylor and Yaktine1). An estimated S-25(OH)D concentration of 50 nmol/l is thought to be sufficient to cover the physiological needs of vitamin D in relation to bone health in 97·5 % of the population(Reference Ross, Taylor and Yaktine1).
Consumption of vitamin D-rich foods
We looked at the prevalence of recommended consumption of major dietary sources of vitamin D only among the immigrant groups (i.e. Maamu) because dietary data collection methods used in the two studies were different and not directly comparable. Dietary data were obtained through non-validated dietary questions asked during the interviews in Maamu, and the questions estimated food consumption frequencies (not amounts) and eating habits(Reference Castaneda, Rask and Koponen20). With a lack of data on consumed amounts, the total dietary intake could not be calculated. During the interviews, the consumption of vitamin D-fortified fat spread was assessed based on the used type (either low-fat spreads [<65 % fat] or ≥65 % fat spreads) and dichotomised into use (‘yes’) and non-use (‘no’). The use of any of the two types of fat spreads was considered as ‘yes’. For fish consumption, participants were asked: how often have you eaten fish or fish dishes during the previous month? The answer to the question was categorised either <2 times per week or ≥2 times per week following the nutrition recommendation of 2–3 times a week(2,31) . The consumption of vitamin D-fortified fluid milk products was examined by the question: how often have you consumed fluid milk products such as milk, sour milk or yoghurt during the previous month? The response was dichotomised into less than daily and daily or several times per day.
Statistical analysis
All statistical analyses were conducted using the Statistical Analysis System for Windows, version 9.4 (SAS Institute Inc.). In all the analyses, inverse probability weights (IPW)(Reference Robins, Rotnitzky and Zhao32,Reference Härkänen, Kaikkonen and Virtala33) , based on the register information on age, sex and study group, were used to reduce bias due to non-response and different sampling probabilities. Finite population correction was used in all the analyses, due to a significant proportion of the total population that was included in the sample(Reference Lehtonen and Pahkinen34). Descriptive statistics were calculated according to study groups and sex. Age-adjusted means and their 95 % CIs for the continuous variable (S-25OHD) were calculated using linear regression. Analyses were done in different sub-populations as described in Fig. 1. Differences between groups were examined with linear (continuous variables) and logistic (categorical variables) regressions. The selected variables as potential determinants of vitamin D deficiency (S-25OHD <30 nmol/l) and insufficiency (<50 nmol/l) among the persons of Russian, Somali and Kurdish backgrounds were identified from previous literature(Reference Granlund, Ramnemark and Andersson15–Reference Jääskeläinen, Knekt and Marniemi17,Reference Thuesen, Husemoen and Fenger35–Reference Madar, Stene and Meyer42) .
In model 1, we adjusted the data for age, season of blood sampling and immigrant group. In model 2, all other characteristic variables (sex, immigrant group, education, BMI, daily smoking, alcohol consumption, physical activity, use of vitamin D supplements, consumption of vitamin D-fortified fat spread, fish consumption and consumption of vitamin D-fortified fluid dairy products) were included in addition to model 1. The results are presented as ORs with 95 % CIs. P-values <0·05 were considered statistically significant. Analyses comparing the immigrant groups with the Finnish reference group (i.e. for participants’ characteristics and vitamin D status) were restricted to participants aged 30–64 years due to limited available data on S-25(OH)D concentration among Finnish younger adults (aged 18–29 years). All other analyses carried out among the immigrants included individuals aged 18–64 years.
Results
Participants’ characteristics
Somali and Kurdish participants were younger compared with Russians and the general Finnish population (Table 1). Somali women had the lowest (14 %) and Russian women the highest proportion of high school education (83 %). Almost half of Somali women and one-third of Kurdish women were obese. The lowest prevalence of obesity was observed among Somali men (5 %). A low prevalence of obesity and a higher proportion of physical activity were observed among both Russian and Finnish participants. The proportions of physical activities were significantly lower among Somalis (18 %) and Kurds (21 %) than in the Finnish reference group. The proportion of daily smokers was low among Somali men (4 %) and Kurdish women (4 %), and similar among Russian and Finnish men but not among women (Russian and Finnish women: 9 v. 17 %, respectively). Smoking was not reported among Somali women. The percentage of excess alcohol consumption was lower in both Russians (10 %) and Kurds (3 %) than among Finns (27 %). No alcohol consumption was reported among Somali men and women. The proportion of vitamin D supplement use was lower in all the immigrant groups, especially among women (10 % Russians, 12 % Somalis and 15 % Kurds) than among the Finnish participants (24 % women). The majority of blood samples among Russians and Kurds were taken during winter, and these differed from that among Finns. Blood samplings among Somalis were almost equally distributed between summer and winter seasons and did not differ from that among Finns.
Table 1 Characteristics of the study population (30–64 years old): immigrant groups (Maamu) and the general Finnish population (Health 2011 Survey)*

AUDIT, Alcohol Use Disorders Identification Test.
* Crude n. Weighted prevalence. P-values from t-tests = differences between immigrant groups and the general Finnish population. Bolded P-values (<0·05) represent statistically significant differences.
† Education (in any country): less than high school = had not received formal school education or attended either primary school or the equivalent/part of primary school and secondary school or equivalent/part of junior high school; high school or equivalent = completed high school or part of high school or an equivalent school. (None of Finnish and Russian men and women reported no educational attainment.)
‡ Excess alcohol consumption: no = AUDIT <6 points for men and <5 points for women; yes = AUDIT ≥6 points for men and ≥5 points for women. (None of Somali men and women reported excess alcohol consumption.)
§ Physically activity: inactive = mainly non-strenuous activities such as reading and watching TV; moderately active = moderately strenuous activities such as walking, biking or light gardening; highly active = strenuous activities such as fitness training or competitive sports several hours a week. NA = not applicable (too few observations for statistical analysis in the cell ‘yes’ for smoking and alcohol consumption). Missing information: for Russians – education (n 5), smoking (n 4), alcohol consumption (n 17), physical activity (n 4), use of vitamin D supplements (n 13); for Somalis – education (n 17), smoking (n 22), alcohol consumption (n 50), physical activity (n 50), use of vitamin D supplements (n 53); for Kurds – education (n 10), BMI (n 4), smoking (n 9), alcohol consumption (n 25), physical activity (n 11), use of vitamin D supplements (n 28); for Finns – education (n 10), BMI (n 2), smoking (n 11), alcohol consumption (n 17), physical activity (n 14), use of vitamin D supplements (n 19). Maamu, Migrant Health and Wellbeing Study.
S-25-(OH)D concentrations and vitamin D status
Lower mean of S-25(OH)D concentrations were found among Kurds (35 (95 % CI 34, 37) nmol/l) and Somalis (44 (95 % CI 41, 46) nmol/l) compared with Finns (64 (95 % CI 62, 66) nmol/l; P < 0·001) (Fig. 2). Russians had similar mean S-25OHD concentrations (64 (95 % CI 62, 66) nmol/l) compared with Finns (P > 0·05; Fig. 2). There were no differences in mean S-25(OH)D concentrations between men and women in any of the study groups (P > 0·05, in all cases). According to IOM thresholds for S-25(OH)D concentrations(Reference Ross, Taylor and Yaktine1), significant differences existed between each immigrant group and the general Finnish group in each of the S-25OHD concentration threshold level (P < 0·001 for all). More than one-fifth of Somali participants and almost half of Kurdish participants were vitamin D-deficient (S-25(OH)D <30 nmol/l) (Fig. 3). The proportions of participants with deficient status were low among Russians and Finns (4 and 1 %, respectively). Insufficient vitamin D status (S-25(OH)D 30–50 nmol/l) was observed in one-fourth of Russians, in more than half of Somalis, and in two out of five Kurdish participants. Only a few of the Finnish participants had insufficient vitamin D status (6 %). In addition, a number of Russian (n 2 (0·5 %)) and Finnish (n 4 (0·5 %)) participants had S-25(OH)D concentrations >125 nmol/l (data not shown). None of Somalis or Kurds had high 25(OH)D concentrations (data not shown).
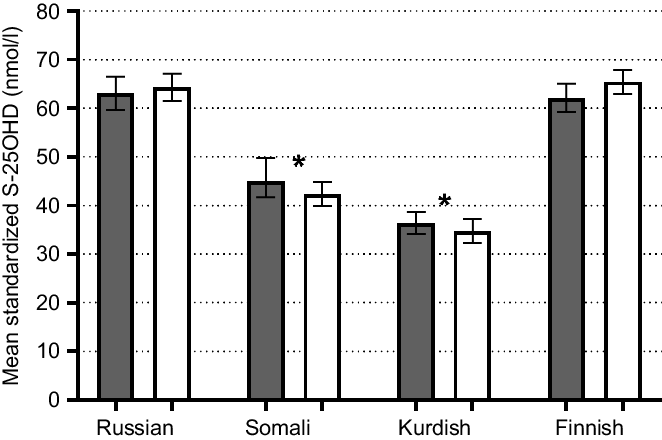
Fig. 2 Weighted mean and 95 % CI of standardised S-25(OH)D concentrations, adjusted for age and month of blood sampling. *P-values from t-tests (<0·001) for mean standardised S-25(OH)D differences between immigrant groups and the general Finnish population. S-25(OH)D, serum 25-hydroxyvitamin D
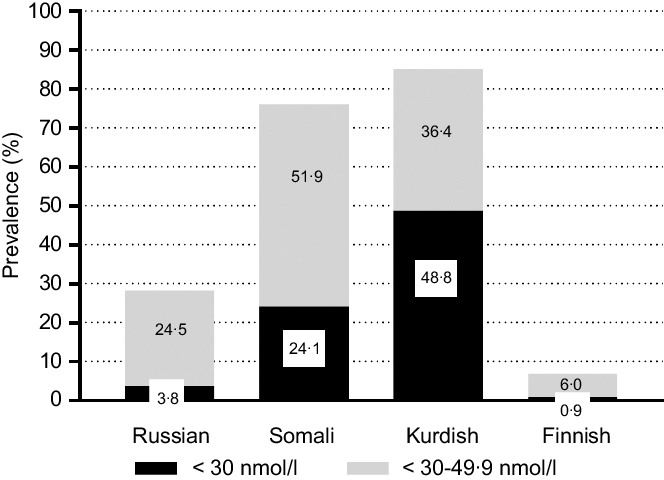
Fig. 3 Weighted prevalence of vitamin D status (S-25(OH)D) <30 and <50 nmol/l for immigrant groups and the general Finnish population, adjusted for age and month of blood sampling. *P-values from t-tests (<0·001) for differences between immigrant groups and the general Finnish population
Consumption of vitamin D-rich foods among immigrant groups
Differences existed in the consumption of vitamin D-rich foods among immigrant groups (Fig. 4a–c). The frequent use of vitamin D-fortified fat spread was observed among a higher proportion of Somalis (91 %) compared with Russians (73 %) and Kurds (60 %; P < 0·001) (Fig. 4a). Fish consumption according to the nutrition recommendation (at least two times a week) was less common among Kurds (17 %) than Russians (43 %) and Somalis (38 %; P < 0·001) (Fig. 4b). More than half of the Russian (57 %) and Kurdish (56 %) participants consumed vitamin D-fortified fluid dairy products daily, whereas the proportion was only one-third among Somalis (36 %; P < 0·001) (Fig. 4c).

Fig. 4 (a–c) Weighted prevalence and 95 % CI of consumption of vitamin D-rich foods among persons of Russian, Somali and Kurdish backgrounds. *P-values from t-tests (<0·001) for differences among immigrant groups
Determinants of vitamin D deficiency among immigrant groups
When comparing only 18–64-year-old immigrants, the proportions of participants with S-25(OH)D <30 nmol/l were 4 % for Russians, 30 % for Somalis and 51 % for Kurds (Table 2). Since the findings of S-25(OH)D <30 nmol/l were low among Russians, the results presented in this section are only for Somalis (n 102) and Kurds (n 252). In the final multivariable model (model 2), participants of Kurdish origin were at a higher risk of vitamin D deficiency compared with Somalis (P < 0·001). Obesity and daily smoking were independent determinants of vitamin D deficiency (P ≤ 0·04, for both). Oldest age group (45–64 years), being physically active and using vitamin D supplements were associated with lower odds of vitamin D deficiency (P ≤ 0·04, for all). The consumption of vitamin D-fortified fat spread and daily consumption of vitamin D-fortified fluid dairy products were also associated with reduced odds of vitamin D deficiency (P ≤ 0·004, for both). Sex, education, alcohol consumption and fish consumption were not associated with vitamin D deficiency (P ≥ 0·06, for all).
Table 2 Association between 18–64-year-old immigrants’ characteristics and vitamin D status (S-25(OH)D <30 and <50 nmol/l)*

* Crude n. ORs and 95 % CIs from logistic regression. P-values from t-tests, <0·05 represents significant associations.
† Results presented only for Somalis and Kurds.
‡ Total number of participants.
§ Number of participants with S-25(OH)D <30 or <50 nmol/l.
|| Model 1 included variables of interest plus age, season of blood sampling and immigrant group.
¶ Model 2 included model 1 plus all other characteristics. NA = not applicable (few observations of S-25(OH)D <30 nmol/l among Russians). S-25(OH)D, serum 25-hydroxyvitamin D.
Determinants of vitamin D insufficiency among immigrant groups
The proportions of participants with S-25(OH)D <50 nmol/l were 29 % (n 128) for Russians, 78 % (n 278) for Somalis and 86 % (n 428) for Kurdish (Table 2). Vitamin D insufficiency was less common among Russians compared with Somalis (P < 0·001). The final multivariable model (model 2) showed daily smoking, excess alcohol consumption and blood sampling in winter season as the significant determinants of vitamin D insufficiency (P ≤ 0·03 for all). Older age (45–64 years), physical activity, fish consumption according to the recommendation and daily consumption of vitamin D-fortified fluid dairy products were associated with lower odds of vitamin D insufficiency (P ≤ 0·04 for all). Sex, education, BMI, use of vitamin D supplements and consumption of vitamin D-fortified fat spread were not associated with vitamin D insufficiency (P ≥ 0·1 for all).
Discussion
The distribution of vitamin D status has been well characterised in the general Finnish population over the last few decades(Reference Spiro and Buttriss5,Reference Lamberg-Allardt, Brustad and Meyer14) , with the latest studies on representative samples of Finnish adults showing sufficient vitamin D status (S-25(OH)D >50 nmol/l) among the majority (77–91 %)(Reference Raulio, Erlund and Männistö18,Reference Jääskeläinen, Itkonen and Lundqvist19) . In contrast, there has only been a limited number of studies of vitamin D status in immigrants in Finland(Reference Islam, Viljakainen and Kärkkäinen12,Reference Adebayo, Itkonen and Öhman13) , and these were with relatively small and largely non-representative samples.
The present study, the first to examine the prevalence of low vitamin D status and its determinants among a representative sample of persons of Russian, Somali and Kurdish backgrounds living in Finland to our knowledge, showed that vitamin D status differed markedly between the subgroups. Mean S-25(OH)D concentrations were significantly (P < 0·001) lower among persons of Kurdish and Somali origins (35 and 44 nmol/l, respectively) compared to both participants of Russian origin and Finnish general population (64 nmol/l for both groups). Not surprisingly, there was a high prevalence of vitamin D deficiency (S-25OHD <30 nmol/l) among persons of Kurdish and Somali origins (48 and 24 %, respectively), with only 4 and 1 %, respectively, among those of Russian origin and Finns. An analysis of determinants showed that being of Kurdish origin, obesity and daily smoking increased the odds of vitamin D deficiency, whereas increasing age, high physical activity, use of vitamin D supplements, consumption of vitamin D-fortified fat spread and fluid dairy products were associated with reduced odds of deficiency. Likewise, there was a relatively high prevalence of Kurdish and Somali participants with S-25OHD >30 but <49·9 nmol/l (36 and 52 %, respectively), with corresponding prevalence of 25 % among those of Russian origin and only 6 % among Finns. Smoking and excess alcohol consumption increased the odds of vitamin D insufficiency (<50 nmol/l), whereas being of Russian origin, increasing age, high physical activity and frequent consumption of vitamin D-fortified fluid dairy products and fish were associated with lower odds of insufficiency.
Our findings from these representative samples of immigrant and Finnish adults in Finland support those of a previous smaller non-representative study that assessed S-25(OH)D concentrations among children and pregnant women in Finland and in the neighbouring Karelian Republic of Russia(Reference Viskari, Kondrashova and Koskela43), as well as those of two earlier smaller studies among immigrants in Finland(Reference Islam, Viljakainen and Kärkkäinen12,Reference Adebayo, Itkonen and Öhman13) . Lower S-25(OH)D concentrations have also been reported among Somali immigrants compared with the Swedish native population(Reference Thuesen, Husemoen and Fenger35,Reference Fernell, Barnevik-Olsson and Bågenholm44,Reference Sääf, Fernell and Kristiansson45) . While immigrants (especially non-Westerners) have been acknowledged widely as being at an increased risk of poor vitamin D status(Reference Van Der Meer, Middelkoop and Boeke4–Reference Lips and de Jongh6), our study clearly shows that the degree of such risk is not the same among all immigrant groups and differs according to the country of origin. For this reason, immigrants should not be considered as one group with similar health behaviours and challenges in terms of vitamin D status, especially when formulating public health policies.
The analysis of determinants of risk of vitamin D deficiency and insufficiency among immigrant groups provided important insights into the means of reducing the risk of low status. For example, beyond the widely reported effect of winter season on vitamin D status(Reference Yao, Hong and Bandera16,Reference Thuesen, Husemoen and Fenger35,Reference Engelman, Meyers and Iyengar36) , in terms of modifiable factors of increased risk of vitamin D deficiency and/or insufficiency, obesity, smoking and excess alcohol consumption were highlighted. While the observed inverse associations between S-25(OH)D concentrations and BMI as well as smoking are consistent with that reported in previous studies(Reference Adebayo, Itkonen and Öhman13,Reference Yao, Hong and Bandera16,Reference Jääskeläinen, Knekt and Marniemi17,Reference Thuesen, Husemoen and Fenger35,Reference Touvier, Deschasaux and Montourcy37–Reference Kassi, Stavropoulos and Kokkoris40) , the observed association between excess alcohol consumption and vitamin D insufficiency is in contrast with positive associations observed between alcohol intake and S-25(OH)D concentrations in previous studies(Reference Thuesen, Husemoen and Fenger35–Reference Touvier, Deschasaux and Montourcy37). The reasons for this are unclear.
While the use of vitamin D supplements and consumption of vitamin D-fortified fat spread and fluid dairy products were not surprising predictors of reduced risk of vitamin D deficiency and/or insufficiency, increasing age and high physical activity were more unexpected determinants. For example, while aging is commonly associated with lower S-25(OH)D concentrations(Reference Touvier, Deschasaux and Montourcy37,Reference Mazahery and von Hurst38) , possibly due to a reduction in the ability of our skin to synthesise vitamin D upon exposure to UVB-rich sunlight(Reference Ross, Taylor and Yaktine1), our result is in line with a recent report of a positive correlation between age and S-25OHD concentration among immigrants in northern Sweden(Reference Granlund, Ramnemark and Andersson15). These findings may be explained by a higher consumption of vitamin D-rich foods and use of vitamin D supplements among older participants. A biological explanation for a positive relationship between S-25(OH)D concentration and being more physically active is not clear(Reference Yao, Hong and Bandera16,Reference Thuesen, Husemoen and Fenger35,Reference Looker41) ; however, such an association may be explained, at least in part, by greater outdoor activities in summertime and thus sun exposure.
In Finland, fluid milk products and fat spreads are fortified with vitamin D(46,Reference Itkonen and Lamberg-Allardt47) , a food fortification policy which has played a major role in eliminating the prevalence of vitamin D deficiency in the general Finnish population(Reference Raulio, Erlund and Männistö18,Reference Jääskeläinen, Itkonen and Lundqvist19) . The predominant contributing dietary source to vitamin D intakes among Finns was milk products, followed by fat spreads and fish dishes(Reference Raulio, Erlund and Männistö18). According to Jääskeläinen et al.(Reference Jääskeläinen, Itkonen and Lundqvist19), vitamin D intakes from diet among the general Finnish population in Health 2011 Survey were 14 and 12 µg/d for men and women, respectively. In the present study, we examined the consumption of most important dietary vitamin D sources among immigrants. A higher proportion of Somalis used vitamin D-fortified fat spreads than participants of Russian and Kurdish backgrounds, whereas the proportion of participants who frequently consumed fish was higher among Russians and Somalis than among Kurds. Daily consumption of vitamin D-fortified fluid dairy products was more common among persons of Russian and Kurdish origins compared with Somalis. Overall, the association of reduced odds of vitamin D insufficiency with the consumption of vitamin D-fortified fluid dairy products and fish among immigrants in the present study is in line with similar findings in a study among immigrants in Sweden(Reference Granlund, Ramnemark and Andersson15).
We found the use of vitamin D supplements to be associated with lower odds of vitamin D deficiency, in line with the findings of other studies of immigrants elsewhere(Reference Granlund, Ramnemark and Andersson15,Reference Madar, Stene and Meyer42) . However, a low proportion of immigrants in the present study were users of vitamin D supplements (9–12 %), which is also in line with that reported in other studies of immigrants, especially non-Westerners(Reference Lips and de Jongh6,Reference Andersson, Björk and Kristiansson9,Reference Wändell11,Reference Islam, Viljakainen and Kärkkäinen12) . The present study showed that 19 % of the general Finnish population used vitamin D supplements.
Strengths and limitations
A key strength of our study was the relatively large number of subjects included, both immigrants and the general Finnish population as a reference group, derived from national representative samples conducted in the same timeframe and with the same protocol. In addition, the use of VDSP data enhanced the quality of our comparison of vitamin D status between the studied immigrant groups and general population. The availability of a wide range of data on potential determinants of vitamin D status is another particular strength of this study. Importantly, data collection in the participants’ mother tongue increased the accuracy of our results. In terms of limitations, the consumption of vitamin D-rich foods may not have been comprehensively assessed through the non-validated dietary questions used among immigrant groups (Maamu). The dietary questions were structured to measure food consumption frequencies and eating habits. Hence, we could not evaluate major dietary sources of vitamin D based on actual intakes. Differences in the dietary data collection methods used in Maamu and Health 2011 Survey(Reference Männistö, Virtanen and Mikkonen48,Reference Paalanen, Männistö and Virtanen49) limited our ability to compare the consumption of vitamin D-rich foods among immigrants with that of the Finnish reference group. The possible effects of skin type, clothing habits and sun exposure (including journeys abroad) as part of the factors associated vitamin D status(Reference Bouillon, Marcocci and Carmeliet7,Reference Granlund, Ramnemark and Andersson15) were not assessed in this study, which might have affected our results on S-25(OH)D concentration. However, most Somali women used covering clothes. We assumed that differences in skin type between Russians compared with Somalis and Kurds contributed to the vitamin D status of each immigrant group. Generally, Caucasians such as Finns and Russians have light skin, while the skin types of Western Asians, like the Kurds, and Africans, such as Somalis, are intermediate and dark, respectively.
Conclusions
Using representative samples, this study confirmed differences in vitamin D status between immigrants, especially among the non-fair-skinned and the native or general European populations. In studies on vitamin D status in different immigrant groups, the learned or existing cultural behaviours should be acknowledged as they are partly associated with most important determinants of vitamin D status. To attain better vitamin D status, adherence to the existing recommended vitamin D intake (dietary and/or supplemental) is highly important for non-fair-skinned immigrants in Northern countries.
Acknowledgements
Acknowledgements: This study used data from Maamu and Health 2011 Survey conducted at the Finnish Institute for Health and Welfare (THL). Financial support: This work was supported by funding received from the Food and Health Doctoral Programme, University of Helsinki. The standardisation of S-25(OH)D data from Maamu was supported by a funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 613 977 (ODIN). The funders had no input in the design, implementation, analysis and interpretation of data. Conflict of interest: None. Authorship: F.A.A., S.T.I., M.E. and C.L.-A. conceived the study. F.A.A., S.T.I., E.L., T.J., A.L., T.L., P.K., M.E. and C.L.-A. contributed to the study design. T.L. and P.K. were involved in the planning and data collection of Maamu. T.J., A.L. and P.K. were involved in the Health 2011 Survey’s data collection. K.D.C. was responsible for the standardisation of S-25(OH)D data. F.A.A. performed statistical analysis with guidance of E.L. Evaluation of results was done by S.T.I., E.L., T.J., A.L., T.L., P.K., K.D.C., M.E. and C.L.-A. F.A.A. interpreted the results and wrote the paper. Critical reviews of the manuscript were done by all co-authors. All authors revised and approved the final manuscript. Ethics of human subject participation: Maamu and Health 2011 Survey were approved by the Coordinating Ethics Committee of Helsinki and Uusimaa Hospital District. The studies were conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all subjects.










