Women's micronutrient status is of particular importance since it affects not only their own health, but also the health of their children( Reference Bartley, Underwood and Deckelbaum 1 ). Deficiencies of iodine and Fe remain major public health problems, affecting >30 % of the global population( 2 , 3 ). Insufficient iodine intake as well as excess iodine intake may cause thyroid diseases( Reference Laurberg, Pedersen and Knudsen 4 ). Fe deficiency is the most common and widespread micronutrient deficiency worldwide( 3 ) and may have multiple adverse effects on thyroid metabolism( Reference Zimmermann 5 ).
Refugees from Western Sahara have been settled in the Algerian desert since 1975 and they are totally dependent on food aid in the harsh, resource-poor desert environment. The refugee population is experiencing a number of challenges related to their food, nutrition and health situation( 6 ). Endemic goitre has been reported among Saharawi schoolchildren and this is probably caused by iodine excess( Reference Pezzino, Padova and Vigneri 7 – Reference Henjum, Barikmo and Gjerlaug 10 ), but further studies are required to understand the aetiology. The main objective of the present paper is to assess iodine status (thyroid volume (Tvol) and urinary iodine concentration (UIC)) and their determinants in Saharawi refugee women of childbearing age. The secondary objective is to assess their prevalence of anaemia.
Methods
Subjects
A cross-sectional survey was performed in January and February 2007 in four Saharawi refugee camps near Tindouf in the Algerian desert. The total population was estimated to be approximately 165 000 persons. Women (15–45 years old) were the target group. We calculated the sample size based on an estimated goitre prevalence of 50 % and an absolute precision of ±5 % for the 95 % confidence interval. This resulted in a required sample size of approximately 400 women, as determined with the EpiInfo Statcalc program version 6·04b (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Each of the four refugee camps was organized into six administrative zones called dairas, and it was assumed that each daira (twenty-four in total) had approximately the same number of inhabitants. To achieve a sample size of 400 women, about seventeen women were included from each daira (400/24≈17). Households in each daira were randomly selected until the required number of women from the daira was obtained. All eligible women in each selected household were included. The average household had four women in the correct age groups, and about four households per daira were needed to reach the required number of women (17/4≈4). The final sample consisted of 394 women from ninety-two households. The response rate was 96 %, and the main reason for not participating was absence on the day of visit. The selection of households was done as follows. The director of health and a representative from the health post in the respective refugee camp, together with a participant from the Norwegian research team, tossed a pen at the centre of the daira and drove towards the border following the pen's direction. At the border a pen was tossed again and the team followed the pen's direction again, driving along both main and small alleys counting adjacent households. Every twentieth household and all its members in eligible age groups were asked to participate in the survey until enough participants were included. If no one was present in the household when the selection team arrived, the next household was visited instead.
Ethical approval for the study was given by the Regional Committees for Medical and Health Research Ethics in Norway and by the Saharawi Health Authorities. Informed written consent was obtained from the chief medical officers in the camps and informed oral consent from the participants in the study. It was emphasised that refusal to participate in the survey would have no negative effects on the woman's entitlements to food aid or other services.
Methods
A portable ultrasound machine (SonoSite Titan®; Vingmed AS, Hovik, Norway) equipped with a 38 mm 5–10 MHz linear transducer was used for the thyroid measurements. Thyroid volumetry was performed according to the method of Brunn et al.( Reference Brunn, Block and Ruf 11 ) and as previously described( Reference Henjum, Strand and Torheim 12 ). To assess thyroid enlargement, two different reference values for Tvol were used: an old reference value where thyroid enlargement was defined as a Tvol exceeding 18 ml( Reference Gutekunst, Becker and Hehrmann 13 ) and a revised reference value where we defined thyroid enlargement as a Tvol exceeding 12·5 ml( Reference Maravall, Gómez-Arnáiz and Gumá 14 , Reference Gómez, Maravall and Gómez 15 ). The revised reference value is based on a healthy, non-iodine deficient Spanish population and mean Tvol (6·5 ml) + 3sd (6·0 ml) in this population was used to define the cut-off of 12·5 ml( Reference Maravall, Gómez-Arnáiz and Gumá 14 , Reference Gómez, Maravall and Gómez 15 ). Spot urine samples were collected from every woman and aliquots were stored at 5°C until analysed. Water samples were collected in every household. Measurement of the iodine concentration in the urine and water samples was performed at the Nutritional Intervention Research Unit in Cape Town, South Africa, by means of ammonium persulfate for the digestion step followed by the Sandell–Kolthoff reaction with microplate reading of the endpoint( Reference Jooste and Strydom 16 ).
Height and weight were measured by standard anthropometric techniques( 17 ). Body weight was measured to the nearest 0·1 kg using a UNICEF electronic weighing scale (SECA 890; Seca, Hamburg, Germany) and height was measured to the nearest 0·1 cm using a UNICEF portable stadiometer. BMI was calculated as weight divided by the square of height (kg/m2). Women were classified as underweight, normal weight, overweight or obese, defined respectively as BMI < 18·5 kg/m2, BMI = 18·5–24·9 kg/m2, BMI = 25·0–29·9 kg/m2 and BMI ≥ 30·0 kg/m2.
Hb concentration was measured from capillary blood samples and read directly in the field by means of a Hb spectrophotometer (HemoCue® 201+; HemoCue AB, Ängelholm, Sweden). Field spectrophotometers were calibrated daily by use of a control cuvette. Anaemia was classified as mild (Hb = 11·0–11·9 g/dl), moderate (Hb = 8·0–10·9 g/dl) and severe (Hb < 8·0 g/dl)( 18 ). The women answered a pre-coded questionnaire concerning marital status, childbirths, education and work. The questionnaire was administered to the women by interview in the local language (Hassania).
Data entry and statistics
Data were entered and analysed using the SPSS statistical software package version 17·0 (SPSS Inc., Chicago, IL, USA). Tvol and UIC did not adhere to a normal distribution; we therefore describe the data by the median and the 25th and 75th percentiles (P25, P75). The data on BMI and Hb were normally distributed and descriptive statistics were therefore reported as mean and standard deviation. Two-tailed tests with a significance level of 5 % were used throughout. All eligible women in each household were included and therefore we used the SVY group of commands for complex survey data in the STATA statistical software package version 10 (StataCorp., College Station, TX, USA) to adjust for clustering within the households. We also adjusted for clustering within dairas.
Tvol, UIC and Hb were dependent variables in multivariate regression analyses. The following technique was used to check for outliers: cases less than Q1 (1st quartile) – 3 × IQR (interquartile range) or larger than Q3 + 3 × IQR were excluded. Univariate regression analyses were used for assessing the association between the dependent variables and selected independent variables. We assessed whether or not the following variables were associated with Tvol: UIC, water iodine, children, breast-feeding, age, BMI, livestock and marital status. For UIC the following variables were assessed: water iodine, breast-feeding, children, age and BMI. For Hb the following variables were assessed: children, breast-feeding, BMI, age and marital status. All covariates showing linear association (P < 0·1) in the univariate regression models were included in a preliminary multivariate regression model. Variables that were still significantly associated in this model (P < 0·1) were retained in the final model. Tvol and UIC did not adhere to a normal distribution and we also undertook the analyses where these dependent variables were log-transformed. Analysis of the residuals was performed in order to examine the fit of the model. In the final model the following interactions between the independent variables were assessed: (BMI × age), (breast-feeding × age) and (number of children × age).
Results
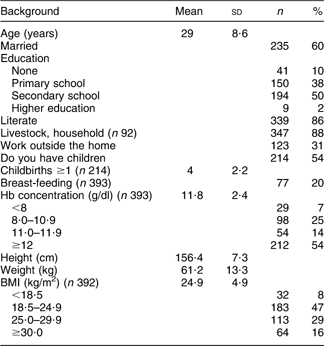
Background and health indicators of the women are presented in Table 1. The mean (sd) age was 29 (8·6) years. More than half of the women (60 %) were married. Fifty per cent had completed secondary school and 86 % were literate. Thirty-one per cent were working outside the home. Fifty-four per cent had children and among those the mean (sd) number of children was 4 (2·2). Twenty per cent were breast-feeding when the survey was undertaken. The mean (sd) Hb level was 11·8 (2·4) g/dl. In total 46 % were anaemic with 14 %, 25 % and 7 % classified with respectively mild, moderate and severe anaemia. The mean (sd) BMI was 24·9 (4·9) kg/m2. Eight per cent were underweight, 47 % were normal weight, 29 % overweight and 16 % were obese.
Table 1 Background and health indicators of Saharawi refugee women (n 394), January–February 2007
The iodine status of the women is presented in Table 2. The median (P25–P75) UIC was 466 (294–725) μg/l. Seventy-four per cent had UIC above 300 μg/l and 46 % above 500 μg/l. The median (P25–P75) iodine concentration in drinking water was 108 (77–297) μg/l. The median (P25–P75) Tvol was 9·4 (7·4–12·0) ml. The value of Gutekunst et al. (old reference value for enlarged Tvol) gave a goitre prevalence of 5 % and the revised reference value a prevalence of 22 %. Sixteen per cent had nodules <1 cm and 5 % had nodules ≥1 cm.
Table 2 Iodine status indicators of Sahrawi refugee women (n 394), January–February 2007
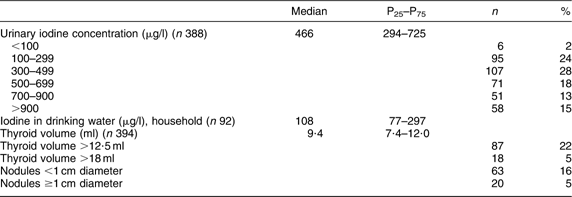
P25, 25th percentile; P75, 75th percentile.
A multiple regression model was used to identify determinants for Tvol. In this model, every kg/m2 increase in BMI resulted in a 0·19 (95 % CI 0·11, 0·27) ml increase in Tvol (P < 0·01). BMI explained 8 % of the variation in Tvol. Determinants for UIC were also assessed in a multiple regression model (Table 3). In the final model, every μg/l increase in water iodine resulted in a 1·32 (95 % CI 0·98, 1·66) μg/l increase in UIC (P < 0·01) and breast-feeding women had on an average 117·0 (95 % CI 49·7, 184·2) μg/l lower UIC than women who were not breast-feeding (P = 0·01). These variables explained 28 % of the variation in UIC and water iodine explained most of the variation. In the model identifying determinants for Hb (Table 4), women who were breast-feeding had 1·10 (95 % CI 0·56, 1·62) g/dl lower Hb concentration (P = 0·01) than women who were not and women with at least one child had 0·12 (95 % CI 0·003, 0·23) g/dl lower Hb concentration (P = 0·04) than women without children. Every kg/m2 increase in BMI was associated with a 0·10 (0·06, 0·15) g/dl increase in Hb concentration (P < 0·01). These three variables explained 8 % of the variation in Hb concentration and BMI explained most of the variation. None of the assessed interactions were statistically significant. The multiple models with log-transformed dependent variables yielded the same significant associations as when we used the untransformed values. We therefore decided to present the data untransformed which are easier to interpret.
Table 3 Determinants of urinary iodine concentration in Saharawi women (n 370Footnote *), January–February 2007
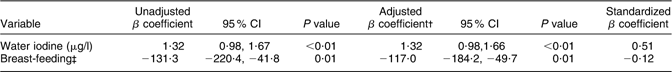
Dependent variable: UIC (μg/l).
* n 394 (five outliers for UIC > 2021 μg/l, six missing data for UIC, twelve missing data for water iodine and one missing data for breast-feeding).
† Adjusted for water iodine and breast-feeding, R 2 = 0·28.
‡ Categories of breast-feeding: 1 = yes, 0 = no.
Table 4 Determinants of Hb in Saharawi women (n 390Footnote *), January–February 2007

Dependent variable: Hb (g/dl).
* n 394 (one missing data for Hb, one missing data for breast-feeding and two missing data for BMI).
† Adjusted for breast-feeding, BMI and children, R 2 = 0·08.
‡ Categories of breast-feeding: 1 = yes, 0 = no.
§ Whether the woman has at least one child, categories: 1 = yes, 0 = no.
Discussion
In the present study we demonstrated that Saharawi women are exposed to excess iodine in drinking water and have unacceptable high levels of UIC. We also confirmed previous findings of high anaemia prevalence in this population.
Urinary iodine concentration
The high median UIC of the Saharawi women indicates that the dietary iodine intake is more than adequate. Ninety per cent of dietary iodine is excreted in the urine, which is a good marker of recent dietary iodine intake in a group( 2 ). The recommended daily intake for adolescents and adults is 150 μg and 250 μg for pregnant and lactating women( 2 ). We found that approximately half of the women had UIC exceeding 500 μg/l. In non-pregnant, non-lactating women, a UIC of 100 μg/l corresponds roughly to a daily iodine intake of about 150 μg under steady-state conditions( 2 ). In our case this corresponds to a daily iodine intake of approximately 750 μg, which is four times higher than the recommended intake. European and US expert committees have recommended tolerable upper intake levels for iodine for adults of 600 μg/d( 19 ) and 1100 μg/d( 20 ), respectively. Excess iodine intake may increase the risk of thyroiditis, hyperthyroidism, hypothyroidism and goitre( Reference Pennington 21 ). However, the health risks associated with iodine deficiency seem to be more severe than those of higher iodine intakes( Reference Laurberg, Andersen and Pedersen 22 ). In healthy adults, short-term iodine intakes of 500–1500 μg/d have been shown to have mild inhibitory effects on thyroid function, probably by inhibiting thyroid hormone release and hormone synthesis( Reference Gardner, Centor and Utiger 23 – Reference Paul, Meyers and Witorsch 25 ). To what extent long-term excess iodine intake may harm the fetus is unknown and of particular importance since our study population was women of childbearing age. One study showed that exposure to excess iodine during pregnancy led to transient hypothyroidism in newborn infants( Reference Clemens and Neumann 26 ).
The main determinants of UIC in the Saharawi women were the iodine level in drinking water and breast-feeding. The median level of water iodine was high (108 μg/l compared with <15 μg/l in tap water) and correlated positively with UIC. We have previously shown that the main dietary sources of iodine among the Saharawi women were drinking water and local milk( Reference Barikmo, Henjum and Dahl 27 ). Iodine-rich drinking water has been reported as a source of excess iodine intake also in China( Reference Li, Liu and Qu 28 ), whereas other studies have reported various sources e.g. seaweed( Reference Suzuki, Higuchi and Sawa 29 ) and iodine-rich meat and milk( Reference Pennington 21 ).
The other determinant of UIC was breast-feeding, where breast-feeding women had a significantly lower UIC than women who were not breast-feeding. Reduced UIC during breast-feeding has been found in other studies( Reference Eltom, Eltom and Elnagar 30 – Reference Torheim, Granli and Sidibé 32 ) and is probably a reflection of the iodine excretion in breast milk( Reference Semba and Delange 33 ).
Thyroid volume
In our study, median Tvol in females (9·4 ml) was slightly higher than Tvol found in other studies from non-deficient areas (6·9–8·9 ml)( Reference Gutekunst, Smolarek and Hasenpusch 34 – Reference Barrère, Valeix and Preziosi 37 ). Larger Tvol has been found in areas with both iodine deficiency( Reference Hegedüs, Perrild and Poulsen 38 – Reference Knudsen, Perrild and Christiansen 42 ) and iodine excess( Reference Li, Liu and Qu 28 , Reference Suzuki, Higuchi and Sawa 29 , Reference Zhao, Chen and Maberly 43 ). Iodine-induced goitre due to iodine-rich drinking water has been found in China( Reference Zhao, Chen and Maberly 43 ). Excessive iodine intakes may lead to thyroid enlargement (goitre), mostly due to increased thyroid-stimulating hormone concentrations( 20 ). A positive association between subclinical hypothyroidism and high iodine intake has been found in several studies( Reference Li, Liu and Qu 28 , Reference Laurberg, Pedersen and Hreidarsson 44 , Reference Szabolcs, Podoba and Feldkamp 45 ). Thyroid hormones were not assessed in our study, but the Saharawi refugees have been exposed to chronic elevated iodine intake for several years, which has probably led to the observed increase in Tvol.
No relationship was found between UIC and Tvol. However, Tvol is an indicator of long-term iodine nutrition, whereas UIC reflects recent intake( 2 ). A relationship between Tvol and UIC is often found when different geographical areas are compared. In contrast, when comparing individuals within an area this relationship is rarely found( Reference Berghout, Wiersinga and Smits 36 , Reference Hegedüs, Perrild and Poulsen 38 , Reference Nygaard, Gideon and Dige-Petersen 39 ). Within a population, a relationship can only be expected if a measure of an individual`s habitual intake is used and the relationship is corrected for other factors that influence thyroid size( Reference Rasmussen, Ovesen and Bülow 46 ).
Two different reference values for enlarged Tvol were used in our study; an old (18 ml) and a revised (12·5 ml). The goitre prevalence was more than four times higher when we used the revised (22 %) compared with the old (5 %). We believe that the revised reference value gives the most correct prevalence of enlarged Tvol for several reasons. First, the data are more recent and based on a population with a long history of adequate iodine intake. Second, high goitre prevalence by palpation (21 %) was found among the Saharawi women in 1998( Reference Pezzino, Padova and Vigneri 7 ). Third, a high prevalence of enlarged Tvol among Saharawi children has been documented in the present study( Reference Henjum, Barikmo and Gjerlaug 10 ) and in previous studies( Reference Pezzino, Padova and Vigneri 7 – Reference Díaz-Cadórniga, Delgado and Tartón 9 ). In conclusion, the women had a high intake of iodine and a high prevalence of goitre and we therefore assume that the Saharawi women are suffering from iodine-induced goitre.
Interpretation of Tvol data requires valid references from iodine-replete populations. However, normal values of Tvol in presumably healthy populations vary depending on the presence of iodine deficiency, and this could lead to a great variation in Tvol to be considered as normal( Reference Maravall, Gómez-Arnáiz and Gumá 14 ). There are no international reference values for enlarged Tvol in women. The old reference value was based on studies in Europe 15–20 years ago. A large proportion living in central Europe at that time was still iodine deficient( Reference Delange 47 – Reference Zimmermann, Hess and Molinari 49 ). Therefore, the Tvol may be higher than what one could expect in a population with sufficient iodine. The revised reference value is based on a randomly selected, healthy, non-iodine deficient Spanish population. However, the revised reference value was based on one local sample. Therefore, larger population studies are required to achieve valid reference data for Tvol in adults.
We found that Tvol was positively associated with BMI. Lean body mass appears to be a major determinant of thyroid size and overweight people have higher lean body mass than normal weight people( Reference Wesche, Wiersinga and Smits 35 ).
Anaemia
Our study confirms findings from a previous study among Saharawi women that demonstrated high prevalence of anaemia( 6 ). We found that 46 % of the women were anaemic; according to WHO, a prevalence of anaemia higher than 40 % is considered as a severe public health problem( 3 ). Breast-feeding, having children and BMI were the main determinants of Hb concentration and explained 8 % of the variation. The women who were breast-feeding probably had lower Hb due to recent delivery and loss of Fe through breast milk. The positive association between BMI and Hb may reflect a higher food intake, and thereby higher intake of Fe in women with higher BMI. Low dietary intake of bioavailable Fe is a major factor in the aetiology of Fe deficiency( 3 ). A study among Saharawi refugee children reported that anaemia was likely due to poor dietary intake( Reference Lopriore, Guidoum and Briend 50 ). In the same study, it was reported that the general food ration was deficient in several micronutrients, particularly Fe, vitamin A and Zn( Reference Lopriore, Guidoum and Briend 50 ). Fe deficiency decreases levels of both triiodothyronine and thyroxine, and reduces thyrotrophin responsiveness, likely through impairment of the haem-dependent thyroid peroxidase enzyme( Reference Zimmermann 5 ). Thyroid function of the women was not measured in our study, but could probably have provided information on how several years of excessive iodine intake and anaemia may have affected thyroid function.
Conclusions
The Saharawi women had high urinary iodine excretion, high levels of water iodine and increased Tvol and probably suffered from iodine-induced goitre. The urinary iodine excretion and water iodine levels have decreased over the past years, but are still high and exceed the recommended intake for the women. The excessive iodine intake may have adverse consequences for the health and development of the Saharawi refugee population and therefore the iodine content of the water has to be further reduced in order to benefit both human and animal health. The continuous high prevalence of anaemia among the women is considered to be a public health concern. To what extent the excessive iodine intake and anaemia have affected thyroid function is unknown and should be addressed in future studies.
Acknowledgements
The work was supported by the Norwegian Research Council (grant number 172226/S30). The authors have no conflicts of interest to disclose. S.H. participated in designing the study and the data collection, and took the lead in analysing, interpreting and writing the manuscript. I.B. and A.O participated in the planning, data collection and writing. T.A.S. and L.E.T. contributed to the study design and analyses, as well as to the writing of the manuscript. All authors read, edited and approved the final manuscript. The authors wish to acknowledge the support provided by Dr Juan José Arrizabalaga and Dr Gurutz Larrañaga Hernando at the hospital Txagorritxu in Vitoria-Gasteiz, Spain. They did the ultrasound training and provided important advice during the fieldwork. They also thank the fieldworkers and the Saharawi families who patiently answered the questions and inquiries.








