Deficiencies of micronutrients are a major public health problem in developing countries. Fe-deficiency anaemia is a worldwide public health problem; global prevalence is estimated at 24·8 % and the highest prevalence occurs in sub-Saharan Africa and south central Asia( 1 ). In the case of breast-fed children aged ≥6 months, complementary food is expected to meet the requirements for almost all micronutrients( Reference Brown, Dewey and Allen 2 ). Although modest information exists on the nutritional status of schoolchildren, studies from several developing countries have demonstrated a high prevalence of micronutrient deficiencies in this age group( Reference Le, Brouwer and Verhoef 3 – Reference Sivakumar, Nair and Sreeramulu 5 ). Undernutrition in general and Fe deficiency in particular among schoolchildren can lead to anaemia and negatively affect growth( Reference Lawless, Latham and Stephenson 6 ), motor and cognitive development( Reference Black 7 ) and immune function( Reference Thurnham 8 ). All of these can adversely affect academic performance( Reference Popkin and Lim-Ybanez 9 ). However, on account of suboptimal feeding practices in terms of quantity and quality, children often tend to receive Fe much below their daily requirement. Therefore an alternative method of providing Fe for this vulnerable segment of the population is required. Among the various strategies, Fe fortification of foods has been suggested as a cost-effective, long-term, population-based strategy with better compliance to improve Fe status and to prevent Fe deficiency worldwide( Reference Allen, de Benoist and Dary 10 , Reference Horton 11 ). Despite several studies during the last 25 years, Fe deficiency still continues as a significant public health problem( Reference Nelson, White and Rhodes 12 ) and its prevention is essential, especially in developing countries( Reference Nelson, Bakaliou and Trivedi 13 ). The prevalence of Fe deficiency in developed countries has declined substantially in the past 15–20 years due to the introduction of fortified foods( Reference Spanjerberg and Jansen 14 ) and other public health programmes such as nutritional advice, emphasis on breast-feeding, education and hygiene( Reference Bhutta, Ahmed and Black 15 – Reference Hallberg 18 ).
In India, limited studies have evaluated the effect of Fe-fortified iodized common salt on Hb levels. The results suggested progressive increase in net Hb concentration in the range of 10–30 g/l after 1 year in rural communities and 5 g/l in urban schoolchildren( Reference Nair 19 ). Thus, there is evidence that children might benefit more from Fe fortification like their counterparts in developed countries.
An array of Fe fortificants suitable for different food vehicles is available. However, the extent of the effect of Fe-fortified foods depends on several factors, like the amount of endogenous Fe in the diet, the amount of Fe fortification, the bioavailability of the Fe fortificant, the food matrix, the frequency of consumption of the fortified food and its duration of feeding, and the Fe status of the individual, as well as the overall nutritional status of the target population( Reference Nelson, White and Rhodes 12 , Reference Hurrell 20 ). The efficacy of Fe fortification strategies is generally evaluated by longitudinal, targeted or population-based, randomized controlled trials (RCT) carried out in a fixed time frame. Fe fortification is considered efficacious when it significantly improves the biomarkers of Fe status and reduces the prevalence of Fe-deficiency anaemia in a population by almost 10 %( Reference Mei, Cogswell and Parvata 21 , Reference Hurrell, Ranum and de Pee 22 ). However, conducting an efficacy trial is costly, invasive and logistically demanding.
In recent years robust study design and meta-analysis have advanced significantly and are readily available( Reference Hedeker and Gibbons 23 ). In the present study, we aimed to systematically review the current literature and to perform a meta-analysis to estimate the effect of Fe-fortified foods on Hb concentration in children. Since we expected heterogeneity among studies, we also explored whether factors such as age, duration of the study and levels of fortification could predict the effects on Hb concentration in children.
Methods
Literature search
The steps in this process were conducted according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines for meta-analysis( Reference Liberati, Altman and Tetzlaff 24 ). We searched both PubMed and the Cochrane Library databases from 1990 up to December 2010, and also reviews and the reference lists of the articles, using the keyword ‘food fortification’ paired with ‘iron’ or ‘hemoglobin’ or ‘dual fortification’ or ‘triple fortification’ or ‘multiple micronutrient fortification’ and ‘fortification trial’.
Selection criteria
The search was regardless of language and publication status. These studies included multiple intervention groups with other micronutrients that were administered simultaneously; the outcome measure was the effect of Fe fortification on Hb concentration only. The studies were limited to publications where RCT evaluated Fe fortification among children aged <10 years for its effect on Hb concentration.
Data extraction and quality assessment
The title and abstracts of the studies identified in the web search were read and irrelevant studies were excluded. Full texts of the remaining studies were retrieved. To avoid publication bias, only peer-reviewed published studies were included. The extraction of data consisted of obtaining sample size, age, duration of intervention, levels of fortification and mean change and standard deviation of Hb concentration in the intervention and control groups. The search, data extraction and quality assessment were completed independently by two content experts according to the inclusion criteria and confirmed by using recommended criteria for RCT( Reference Clarke and Oxman 25 , Reference Juni, Altman and Egger 26 ). Concealment of allocation was classified as ‘adequate’, ‘unclear’, ‘inadequate’ or ‘not used’, based on randomization, blinding and reporting of withdrawals. Blinding was classified as ‘double blinding’, ‘single blinding’, ‘no blinding’ or ‘unclear’. In designs employing two or more different intervention groups (different levels of fortification or administration regimens) and a single control group, the sample size of the control group was equally allotted to the number of intervention groups while retaining the same mean value for the change and its standard deviation. In reporting such designs, each intervention subgroup was analysed separately. Thus, some studies contributed more than one intervention component with a single control group for the statistical analysis and resulted in a greater number of trials than the number of studies included.
Statistical analysis
Our primary outcome was the mean change in Hb concentration on account of consumption of Fe-fortified foods. The effect size, which is the difference in means between the Fe-fortified and the control groups, is referred to as the weighted mean difference (WMD) and was calculated for the selected trials. Once an effect size was estimated for each trial, the overall effect of these results was assessed by the Q statistic, that measures the extent of inconsistency among studies. The Q test was computed under the assumption of homogeneity among the effect sizes and the statistic follows the χ 2 distribution with k−1 degrees of freedom, k being the number of studies. Another strategy for quantifying the heterogeneity in a meta-analysis consists of estimating the variance (τ 2) between studies. The parameter I 2 quantifies the extent of heterogeneity from a collection of effect sizes, which is interpreted as approximately the percentage of total variation in study estimates due to heterogeneity rather than sampling error. The overall WMD of these results was assessed for sampling error (homogeneous, τ 2 = 0). A fixed-effects meta-analysis was applied to obtain the pooled effect size with 95 % confidence interval or else a random-effects meta-analysis was performed (heterogeneous, τ 2 > 0)( Reference DerSimonian and Laird 27 , Reference Borenstein, Hedges and Julian 28 ).
The heterogeneity of results was represented in the form of a forest plot. Typically, for each study, there is a blob in the middle of the 95 % confidence interval that represents the single best mean estimate of the Hb concentration found in that study. The pooled or combined result of the WMD in Hb is represented by a diamond, the width of which is the 95 % confidence interval for the pooled data. A vertical line is displayed to indicate no effect and to differentiate between the studies that favour the intervention group or the control group. The forest plot also describes the χ 2 (Q-test statistic), τ 2, df, I 2, Z and P value. An I 2 value of more than 50 % is considered to indicate significant heterogeneity between the trials( Reference Higgins, Thompson and Deeks 29 ). Publication bias was assessed with the funnel plot and Egger regression test. This is equivalent to a weighted, linear, ordinary least squares regression model with standard error as a covariate( Reference Egger, Davey Smith and Schneider 30 ).
If heterogeneity existed (I 2 > 50 %), a meta-regression approach was used to test the study heterogeneity by relating study characteristics. The confounders were identified and a covariate meta-analysis was performed to estimate the net pooled effect size, after removing the effect of covariates (confounders).
Statistical analyses were performed with Review Manager (RevMan) software version 5·1, IBM SPSS version 19·0 and Comprehensive Meta-Analysis (CMA) software trial version (www.meta-analysis.com).
Results
Search results
A total of 846 articles were identified, of which 779 were excluded because they were not RCT or their interventions were not relevant to the purpose of the current analysis. Sixty-seven potentially relevant articles were selected for full text evaluation, out of which eighteen relevant articles were submitted to meta-analysis after employing the inclusion and exclusion criteria (Fig. 1).
Fig. 1 Flow diagram for inclusion in the present meta-analysis of randomized controlled trials assessing the effect of iron-fortified foods on mean Hb concentration in children (<10 years)
Study characteristics and data quality
Characteristics of the eighteen studies included in the analysis( Reference Bradley, Hillman and Sherman 31 – Reference Sazawal, Dhingra and Dhingra 48 ) are shown in Table 1. All of these were RCT, out of which six were double blind, two were cluster randomized trials and the remaining ten were randomized trials. Of the ten RCT, all had similar Hb concentrations in intervention and control groups at baseline. A total of 5142 children of average age 6 months to 9·5 years, with levels of Fe fortification such that daily Fe intake through fortified food ranged between 3·5 and 12·7 mg per child, with intervention duration ranging between 6 and 24 months, were studied. These studies have been carried out over the past 20 years. Included in the analysis were five studies each from Brazil and India; two each from Vietnam and South Africa; and one each from Indonesia, Kenya, Korea and the USA.
Table 1 Summary of trials assessing the effect of iron-fortified foods on mean Hb concentration in children (<10 years)

B/A, before-and-after study; DB, double blind; RCT, randomized controlled trial; PC, placebo controlled; CRT, cluster randomized trial.
All eighteen studies evaluated the effect of various levels of fortification on Hb concentration. Four studies of multiple interventions with multiple micronutrients were included and only those groups that received Fe were considered( Reference Bradley, Hillman and Sherman 31 , Reference Moretti, Zimmermann and Muthayya 38 , Reference Varma, Das and Sankar 44 , Reference Nga, Winichagoon and Dijkhuizen 46 ). Of these four included studies, each one had more than one trial. The trials were either based on different levels of intervention of food fortification conducted on two occasions (before and after) or they were compared with placebo. In each trial the number of participants, mean and standard deviation of Hb concentration were estimated for conducting meta-analysis.
Effects of Fe fortification on Hb concentration
The meta-analysis results indicated that the mean change in Hb concentration was significantly higher in the Fe-fortified group than in the control group (n 5142; WMD = 5·09 g/l, 95 % CI 3·23, 6·95 g/l; P < 0·00001), as depicted on the forest plot (Fig. 2). There was significant heterogeneity for the mean Hb concentration reported among the included trials. All statistical tests of heterogeneity – such as the Q statistic (χ 2 = 226·03, df = 23), which was more than df; τ 2 greater than zero (τ 2 = 18·37); and I 2 greater than 50 % (I 2 = 90 %) – were higher than the expected value, indicating heterogeneity among the studies. Meta-regression analysis was performed to detect the source of heterogeneity and indicated that the duration of the intake of fortified food was positively related to the effect size (regression coefficient = 0·368, 95 % CI 0·005, 0·731; P < 0·05). The significant differences in the extent of improvement in Hb levels as reported in the forest plot (Fig. 2) are perhaps due to different time periods of the feeding regimens of Fe-fortified foods to the children. Increased duration of receiving fortified foods might have resulted in higher levels of Hb.
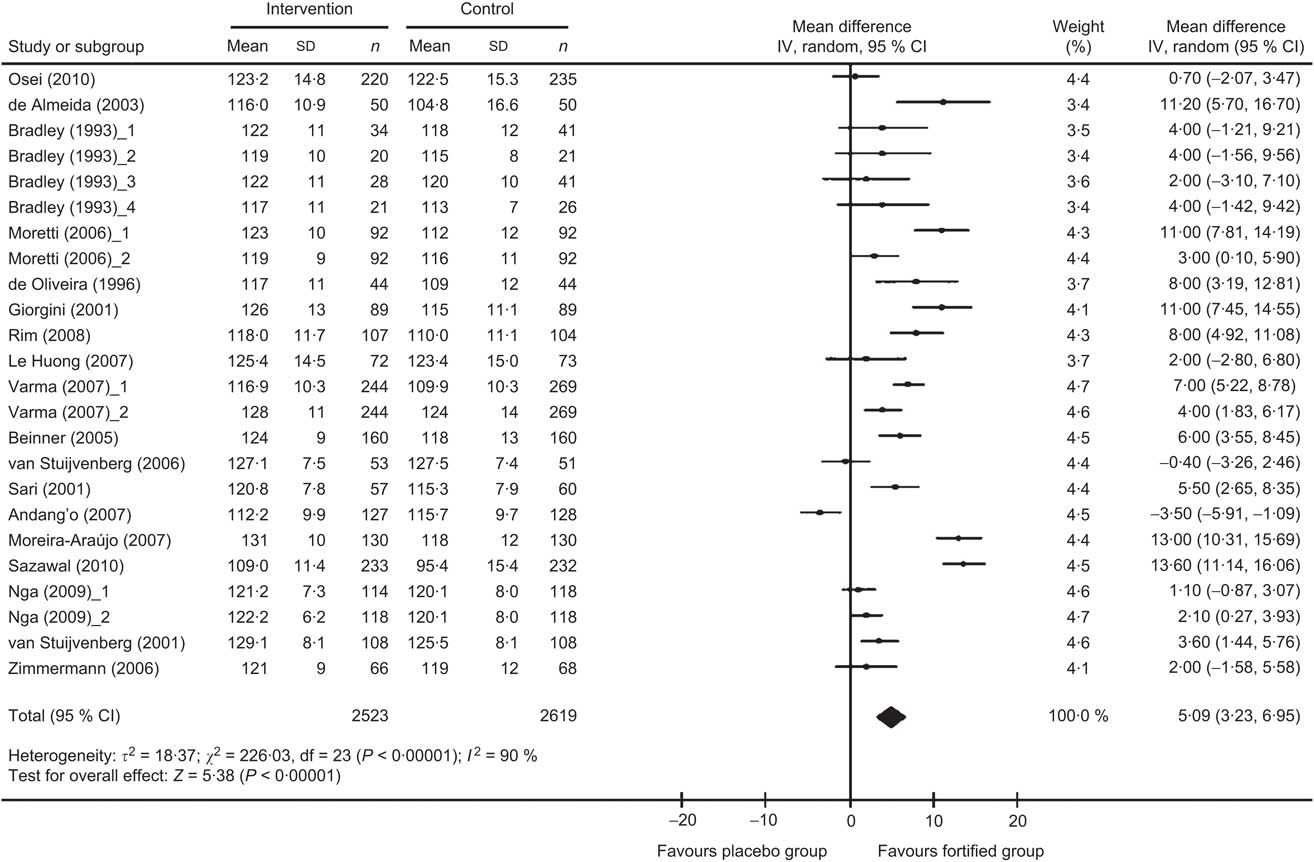
Fig. 2 Forest plot: effect of iron fortification on mean difference in Hb concentration in comparison with no intervention or placebo control in children (<10 years). Random-effects meta-analysis of weighted mean difference (WMD; and 95 % CI) on Hb concentration with iron-fortified food intervention compared with control group. The sizes of data markers indicate the weight of each study in the analysis. Horizontal lines represent 95 % CI. Blob indicates best estimate and diamond indicates the summary estimate of the WMD
Covariate meta-analysis was performed to eliminate the effect of the confounder, duration of the study. Upon removal of the confounders the net effect of fortification on Hb concentration in children was found to be 4·74 (95 % CI 3·08, 6·40) g/l, as compared with the calculated pooled effect size of 5·09 (95 % CI 3·23, 6·95) g/l.
Publication bias
The funnel plot (Fig. 3) was symmetrical, indicating the probable absence of publication bias which was confirmed using Egger's weighted regression method (Egger test, P = 0·6276).

Fig. 3 Funnel plot of all individual studies in the meta-analysis. Studies that evaluated the effect of iron fortification on Hb concentration in children (<10 years) were plotted with their weighted mean difference (WMD) on the x-axis and the corresponding standard error of the WMD along the y-axis
Discussion
The Fe fortification intervention varied across trials; hence, results should be interpreted accordingly. The present meta-analysis of eighteen studies consisting of twenty-four trials found that Fe fortification was significantly associated with increased Hb concentration in intervention children compared with the controls. However, there was heterogeneity in the results across the trials.
The present study adopted sequential statistical methods to verify that implementation of fortified foods with Fe improves Hb concentration in the child beneficiaries. The presence of heterogeneity is an important attribute of meta-analysis that can influence the results and was tested by the Q statistic, τ 2 and I 2, with the results represented in the form of a forest plot. The results of each trial included in the analysis showed the mean difference in Hb concentration (5·09 g/l) favours the intervention group, suggesting that the Fe fortification improves the mean Hb level of children. We found that the value of Q is more than the degrees of freedom, indicating heterogeneity and suggesting that the variation in the mean changes of Hb between intervention and control groups is due to systematic underlying differences( Reference Borenstein, Hedges and Julian 28 , Reference Sterling, Rosenbaum and Weinkam 49 ). One of the limitations of this statistic could be due to the inclusion of studies with n < 30( Reference Bradley, Hillman and Sherman 31 ) and this might have contributed to the heterogeneity. Similarly, the second measure of heterogeneity, τ 2, also indicated that the variance of WMD was more than zero, which confirms that there existed heterogeneity among the trials. A third measure of heterogeneity, the I 2 statistic, which is a derivative of Q, was 90 %, also suggesting heterogeneity among the selected trials( Reference Cook, Guyatt and Ryan 50 , Reference Moher, Pham and Klassen 51 ).
Since there was heterogeneity among the trials, fixed-effects meta-analysis could not be performed and we applied the random-effects meta-analysis. The random-effects meta-analysis showed a significant impact of Fe fortification on Hb concentration among the child beneficiaries and provides evidence to suggest that food fortification with Fe is an ideal strategy to correct Fe-deficiency anaemia among children <10 years of age.
Further, to understand the true effect of food fortification with Fe on Hb concentration, meta-regression analysis was performed to explain the influence of confounders such as age, duration of intervention and levels of fortification( Reference Egger, Zellwerger-Zahner and Schneider 52 ). We observed that the duration of the study is an effective confounder. The covariate meta-analysis showed that the net effect was 4·74 g/l after removing the effect of confounders. Yet another critical step in meta-analysis is the publication bias which can lead to inflated estimates of efficacy. We observed that there was heterogeneity among trials as some of the trials did not fit into the funnel. However, Egger's regression test suggested that there was no publication bias (P = 0·6276).
One concern is related to the unresolved issue regarding an interaction between Fe and infection. As per the review by Oppenheinmer( Reference Oppenheimer 53 ), most adverse effects of Fe supplementation have been reported following the use of parenteral Fe in geographical areas where malaria is endemic. Subsequently, the results of a trial conducted in Pemba, Zanzibar showed an increase in risk of hospitalization and mortality after Fe supplementation among Fe-replete children in a malaria-endemic setting( Reference Sazawal, Black and Ramsan 54 ). A very similar trial in malaria-free areas in Nepal found no such adverse effect( Reference Tielsch, Khatry and Stoltzfus 55 ). However, no adverse effects were reported in the studies covered in present paper where Fe-fortified foods were involved.
There is emerging evidence to suggest that reports of RCT from certain countries mostly have statistically positive results on micronutrients( Reference Vickers, Goyal and Harlan 56 ). This may be due to the fact that in those countries, anaemia is largely due to Fe deficiency (single nutrient) rather than the multifactorial aetiology reported from developing countries. However, the present study suggests the possibility of a positive effect of Fe fortification on Hb in children. This phenomenon needs to be investigated further. There is a need to conduct more trials in developing countries so as to investigate whether Fe fortification improves Hb concentration and also to assess other confounders, such as intakes of protein, energy and folic acid, which could contribute in improving Hb levels.
Conclusion
This present study suggests that consumption of Fe-fortified foods significantly increases the Hb concentration in children aged <10 years. Further research efforts should concentrate on higher quality and more rigorous randomized trials with longer follow-up to resolve the uncertainty regarding the safety and clinical effectiveness.
Acknowledgements
Source of funding: The authors gratefully acknowledge the financial and fellowship support extended by the National Institute of Nutrition (NIN), Indian Council of Medical Research, Hyderabad, India, for carrying out the study. Conflicts of interest: There are no conflicts of interest. Ethics: Ethical approval was not required for the present study. Authors’ contributions: R.A. contributed in the data collection, analysis and manuscript preparation. M.V.V.R. developed the study protocol, secured funds, supervised the study and guided manuscript preparation. K.M.N. contributed to development of the study protocol and manuscript writing. Acknowledgements: The authors thank Dr B. Sesikeran (Director, NIN) for his encouragement, and also Mr K. Venkaiah and Dr N. Bala Krishna (Biostatistics Division, NIN) for their insightful inputs.








