Diabetic kidney disease (DKD) is the leading cause of chronic kidney disease and end-stage renal disease, accounting for an estimated 50 % of all end-stage renal disease cases globally(Reference Tuttle, Bakris and Bilous1,Reference Fu, Liu and Bastacky2) . It has been confirmed that DKD, as one of the most common microvascular complications of diabetes, is associated with considerable morbidity and mortality(Reference Saran, Robinson and Abbott3). In addition, the progression of DKD to end-stage renal failure frequently requires renal replacement therapy, which carries with substantial health care costs(Reference Thomas4). With the rising incidence of diabetes mellitus, threat from DKD will possibly be exacerbated(5). Therefore, it is imperative to uncover additional modifiable factors in addition to the classic factors for DKD, which would be helpful in developing targeted prevention strategies.
The pathophysiological changes of DKD are likely attributable to the metabolic and haemodynamic abnormalities(Reference Ruospo, Saglimbene and Palmer6); however, the exact underlying mechanisms are complex and may involve multiple pathways. DKD is regarded as a metabolic-driven immunological disease(Reference Mosterd, Kanbay and van den Born7). Existing evidence has suggested associating DKD risk with inflammation, indicating that both systemic and local renal inflammation play crucial roles in the development of DKD(Reference Pérez-Morales, Del Pino and Valdivielso8). Diet, as one of modifiable lifestyles, should not be neglected as a potential source of inflammation because of the property of specific nutrient. Existing studies indicated that some anti-inflammatory nutrients, for example, fibre, vitamin D and n-3 PUFA, are associated with lower serum levels of inflammatory biomarkers and lower risk of albuminuria and then slower kidney function decline(Reference Xie, Huang and Cao9–Reference Lee, Oh and Park11). Of note, current clinical guidelines recommend a comprehensive approach examining the effects of overall diet rather than solely looking at individual nutrient, considering that it allows for easier translation into practical dietary advice(Reference Cespedes and Hu12). Therefore, assessing the overall inflammatory potential of diet and understanding the relation of diet-induced inflammation with DKD risk are important, as it may offer a unique perspective to develop strategy to alter dietary habits and harness the onset and progression of DKD.
The dietary inflammatory index (DII) represents a new tool to measure the dietary inflammatory potential(Reference Shivappa, Steck and Hurley13), which has been proved to be associated with inflammation(Reference Shin, Lee and Brann14,Reference Kotemori, Sawada and Iwasaki15) . Accumulating evidence has identified DII to be a potential risk factor for various diseases, such as cancers(Reference Puddu, Shivappa and Menotti16) and diabetes(Reference Laouali, Mancini and Hajji-Louati17), which are risk factors for DKD. However, fewer studied have investigated the association between dietary inflammatory potential and kidney health, and some limitations have been proposed. For example, previous studies only conducted in specific population (e.g. specific age groups)(Reference Xu, Sjögren and Ärnlöv18). Yet, no study has investigated the effect of the dietary inflammatory potential on DKD in a nationally representative sample, and the pattern of dose–response associations remains to be explored.
Because of lack of exhaustive estimate on the relationship, it is imperative to undertake an updated, comprehensive research to bridge the knowledge gap. Accordingly, the present study using data from the National Health and Nutrition Examination Survey (NHANES) aimed to assess if the DII is associated with DKD and to determine if the association presents in a dose–response manner.
Materials and methods
Study sample
The NHANES is a nationally representative survey conducted in the US population, aimed to assess health and nutrition status(Reference Johnson, Paulose-Ram and Ogden19). The periodic surveys, in which a stratified multistage probability sampling method was used, included data regarding demographics, socio-economics, lifestyle habits and laboratory tests. The details of NHANES are available elsewhere(Reference Schorgg, Bärnighausen and Rohrmann20). The written informed consent was obtained from all participants(21).
Data from five NHANES cycles (2007–2016) were incorporated in the present study. Participants aged 20 and under were excluded (n 21 387). We excluded participants who were pregnant or lactating (n 403). According to previous studies, participants with unusual energy intakes of less than 2092 kJ/d (500 kcal/d) or above 20 920 kJ/d (5000 kcal/d) in females and less than 2092 kJ/d (500 kcal/d) or above 33 472 kJ/d (8000 kcal/d) in males (n 3277) were excluded(Reference Wang, Jiang and Wu22). Other participants were excluded for missing values of DKD (n 1121). Remaining 4264 participants with diabetes were included in the analyses. Figure 1 presents the flow chart of study sample.
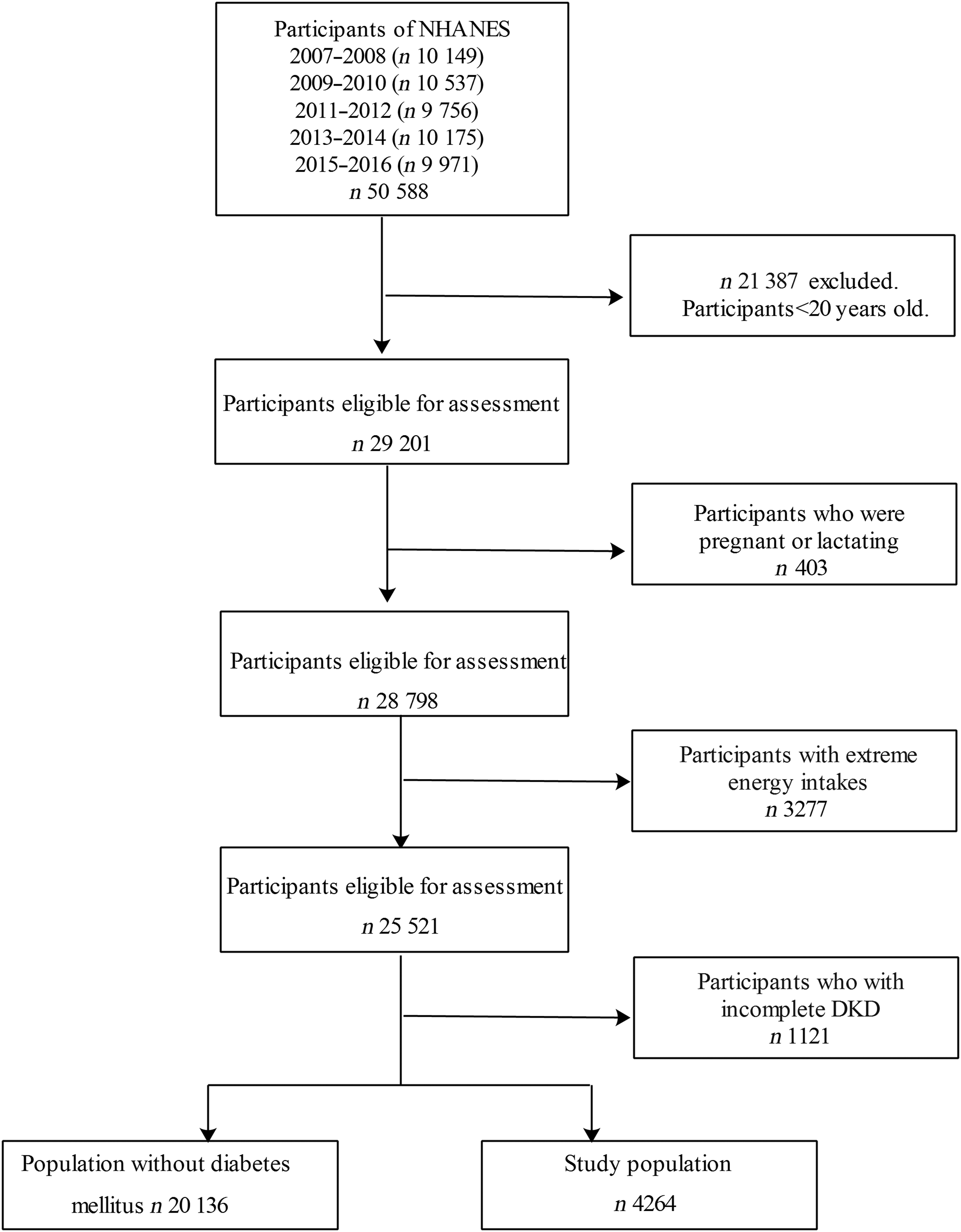
Fig. 1 Flow chart of study sample. NHANES, National Health and Nutrition Examination Survey; DKD, diabetic kidney disease
Definition of diabetic kidney disease
DKD was defined as diabetes with the presence of albuminuria, impaired glomerular filtration rate (GFR) or both(Reference de Boer, Rue and Hall23). Diabetes was defined as (1) a self-reported previous diagnosis by health care professionals, (2) fasting plasma glucose level of 7·0 mmol/l or higher, (3) HbA1c concentration of 6·5 % or higher or (4) taking glucose-lowering medications. Albuminuria was defined as the ratio of urine albumin to creatinine (ACR) of 30 mg/g or higher. Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate the GFR. GFR less than 60 ml/min per 1·73 m2 was defined as impaired GFR.
Assessment of dietary inflammatory index
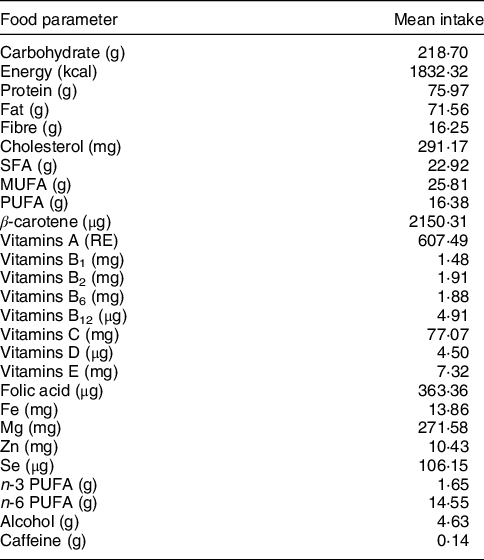
The DII was computed based on the dietary intake data gathered by day 24-h dietary recalls. We calculated the DII score for twenty-seven food parameters available. Table 1 presents the mean intakes of the included food parameters. The calculation of DII was as follows: first a Z-score was calculated by subtracting the ‘standard global mean’ from the reported amount for each food parameter, which were then divided by the sd. The Z value was converted to a percentile score and transformed to a centred score. The corresponding inflammatory effect score multiplied by the above derived values to produce the DII score. A lower DII score represents a more anti-inflammatory diet, whereas a higher DII score represents a more pro-inflammatory diet. In our analyses, DII score varied between −4·73 and 4·55 and categorised into quartiles: quartile 1 (Q1: −4·73, −0·52), quartile 2 (Q2: −0·51, 1·01), quartile 3 (Q3: 1·02, 2·30) and quartile 4 (Q4: 2·31, 4·55).
Table 1 Food parameters included in the dietary inflammatory index
Covariates
The following potential covariates included in the analyses were selected based on literature review and availability in our data set: age, sex, race (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic and other race), educational level (below high school, high school and above), marriage status (married/living with partner, widowed/divorced/separated/never married), family poverty income ratio, smoking status (never, current and former), drinking status (no, yes), physical activity level (low, moderate and high), BMI and hypertension. The physical activity was assessed using the Global Physical Activity Questionnaire. BMI was calculated as weight in kilograms divided by height in meters squared. Participants rested quietly in a sitting position for 5 min, and three consecutive blood pressure readings were obtained and averaged. Hypertension was defined as the average systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of anti-hypertensive medication. All above related information was gathered through standardised questionnaire, physical examination and laboratory tests.
Statistical analysis
Sample characteristics were compared using the ANOVA for continuous variables, and the χ 2 tests for categorical variables. Binary logistic regression models were conducted to examine the association between DII and DKD with OR and 95 % CI, in which the Q1 of DII was the reference category. We firstly adjusted for age, and sex, then for race, educational level, marriage status, family poverty income ratio, smoking status, drinking status, physical activity level, hypertension and BMI in the multivariable-adjusted model. Furthermore, restricted cubic spline models with knots at the 5th, 35th, 65th and 95th percentiles were performed to explore the shape of dose–response relationship between DII and DKD adjusted for all above covariates(Reference Desquilbet and Mariotti24). In addition, stratified analyses were carried out by socio-demographic and clinical characteristics including sex, age (middle-aged adults: < 60 years old, older adults: ≥60 years old), educational level, BMI (underweight/normal: ≤ 24·9 kg/m2, overweight/obese: >25 kg/m2) and status of hypertension. Stata 15.0 software (StataCorp., LP) was used in all analyses.
Results
Among 4264 participants, the weighted proportion of DKD was 36·2 %. The characteristics of our sample across quartiles of DII score are shown in Table 2. Participants in the Q4 of DII score were older, more likely to be female, single, have lower educational level, less physical activity, less household income, higher BMI, current smoking and drinking. The prevalence of hypertension, DKD, impaired GFR and albuminuria was higher among participants with the most pro-inflammatory diet.
Table 2 Characteristics of study sample according to DII quartiles
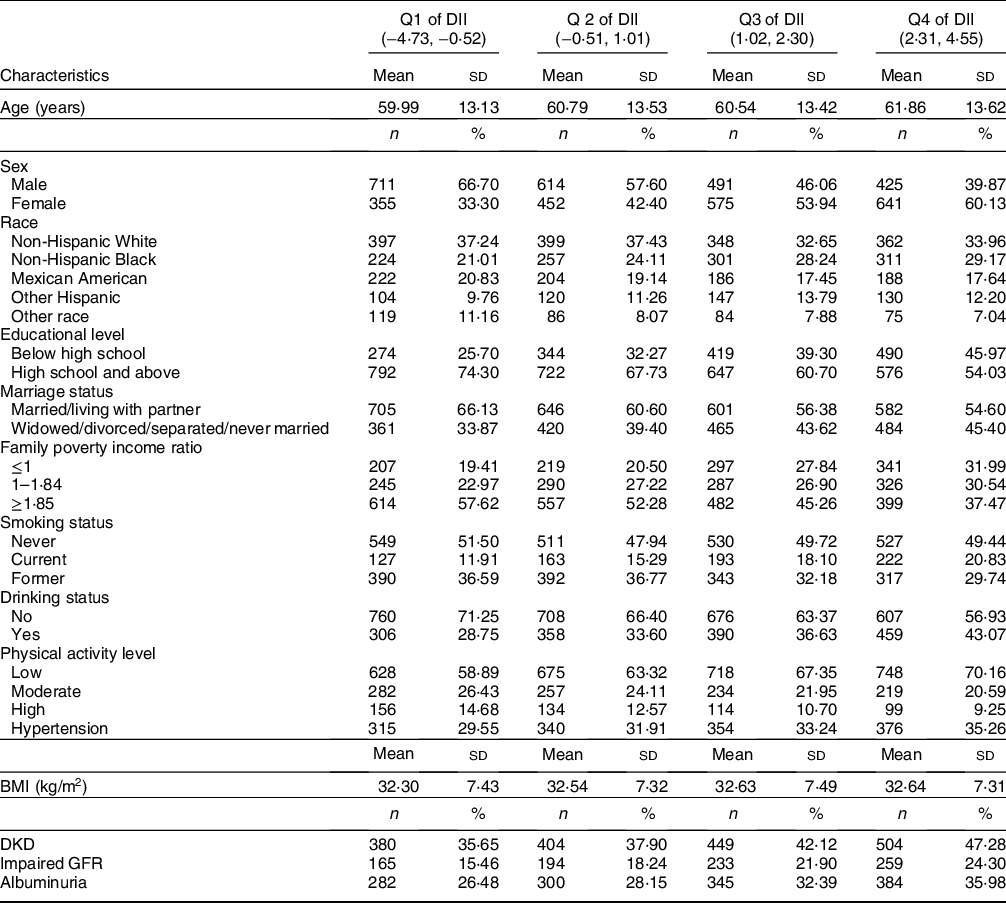
DII, dietary inflammatory index; DKD, diabetic kidney disease; GFR, glomerular filtration rate.
Table 3 shows the weighted associations between DII with DKD, impaired GFR and albuminuria. In the multivariable-adjusted model, the OR of DKD was 1·04 (95 % CI 0·81, 1·36) for the Q2, 1·24 (95 % CI 0·97, 1·59) for the Q3 and 1·64 (95 % CI 1·24, 2·17) for the Q4 compared with Q1 of DII score. Similar figures were 1·16 (95 % CI 0·81, 1·68), 1·35 (95 % CI 0·97, 1·88) and 1·57 (95 % CI 1·10, 2·26) for impaired GFR, and 1·00 (95 % CI 0·75, 1·33), 1·28 (95 % CI 0·94, 1·72) and 1·56 (95 % CI 1·14, 2·12) for albuminuria.
Table 3 Weighted OR (95 % CI) of the association between DII and DKD
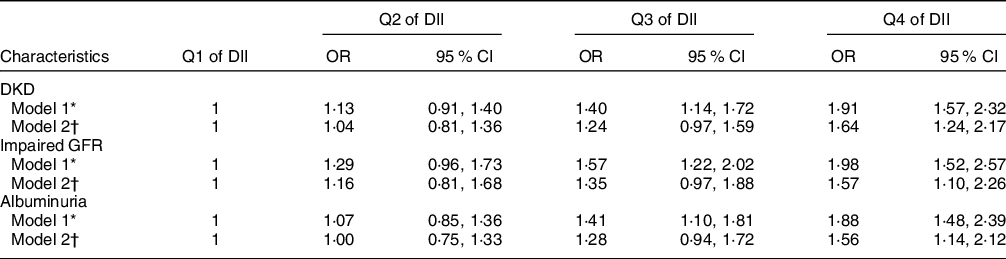
DII, dietary inflammatory index; DKD, diabetic kidney disease; GFR, glomerular filtration rate.
* Model 1 adjusted for age and sex.
† Model 2 adjusted for age, sex, race, educational level, marriage status, family poverty income ratio, smoking status, drinking status, physical activity level, hypertension and BMI.
In the cubic spline model, a linear dose–response relationship was found between DII and DKD (P nonlinearity = 0·73). The adjusted OR for per unit increasing of DII was 1·13 (95 % CI 1·07, 1·19) for DKD. The dose–response relationship between DII and DKD is presented in Fig. 2.
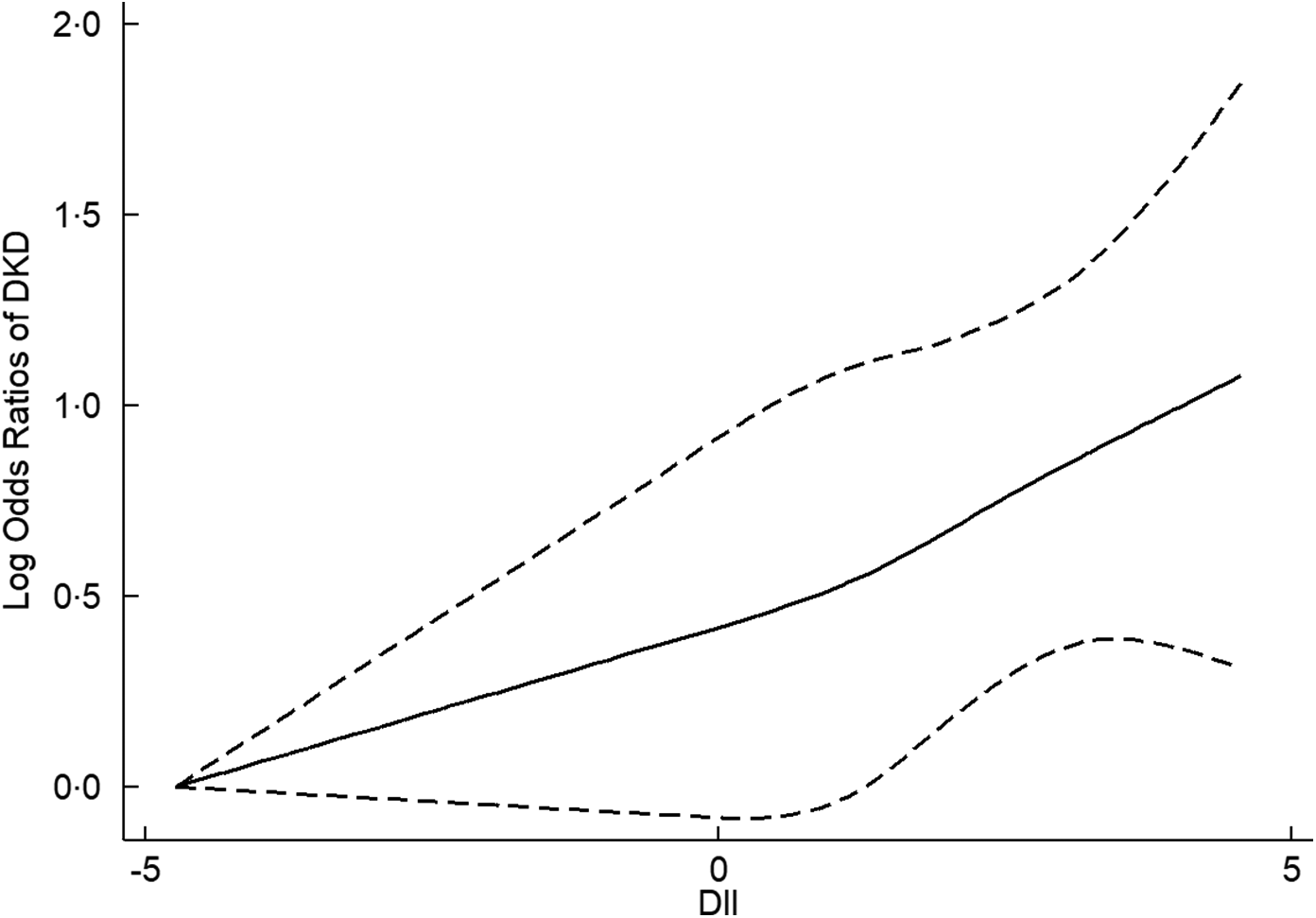
Fig. 2 The dose–response relationship between dietary inflammatory index (DII) and diabetic kidney disease (DKD)
In the stratified analyses, the association of DKD for the Q4 of DII was statistically significant among adults with higher educational level, with OR 1·83 (95 % CI 1·26, 2·66), but not among adults with lower educational level. The association for the Q4 was statistically significant among overweight or obese participants, with OR 1·67 (95 % CI 1·23, 2·28), but not among underweight/normal participants. The interaction effects between DII and stratified factors on DKD were not statistically significant (all P values for interactions were >0·05). The associations of DII with DKD in stratified analyses are shown in Table 4.
Table 4 The weighted OR (95 % CI) of the association between DII and DKD in stratified analyses
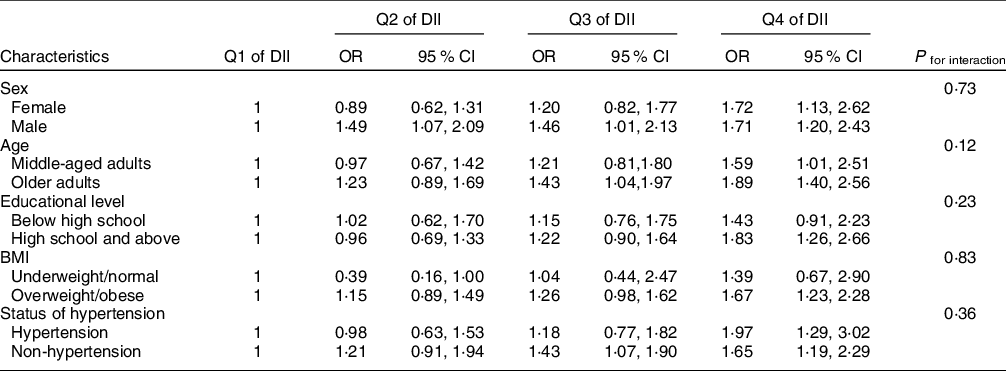
DII, dietary inflammatory index; DKD, diabetic kidney disease.
Models adjusted for age, sex, race, educational level, marriage status, family poverty income ratio, smoking status, drinking status, physical activity level, hypertension and BMI.
Discussion
In the present study, we explored the association between DII and DKD using a nationally representative sample of US adults. A more pro-inflammatory diet, as estimated by a higher DII score, is associated with increased odd of DKD. Particularly important, the association presents in a linear dose–response manner.
The findings were consistent with previous studies on the association between dietary patterns and kidney function(Reference Geng, Jafar and Neelakantan25–Reference Hu, Steffen and Grams27). For example, a prospective analysis in the Nurses’ Health study found that adherence to the pro-inflammatory Western-style diet, compared with a healthier diet, was associated with an increased risk of GFR decline, with the associations no variation by diabetes status(Reference Lin, Fung and Hu28). Conversely, the higher alternative healthy eating index, a measure of diet quality negatively correlated with DII(Reference Wirth, Hébert and Shivappa29), was associated with a lower odd of albuminuria among population with diabetes(Reference Dunkler, Dehghan and Teo30). Similarly, a multi-ethnic study also showed that a diet rich in fruit and wholegrains, with presumed anti-inflammatory properties, was associated with lower odds of micro-albuminuria in individuals with diabetes(Reference Nettleton, Steffen and Palmas31). Although each dietary index represents a unique combination of dietary nutrients, to some extent, they share considerable similarities and have been significantly associated with inflammatory markers(Reference Hart, Torres and McNaughton32). Notably, DII is designed to assess the dietary inflammatory potential, which represents the inflammatory mechanism underlying the diet–health link. Importantly, it is worth emphasising that our findings make a significant addition to literature by demonstrating association between DII and DKD among a nationally representative sample of population. The observed associations emphasise the potential of avoiding pro-inflammatory diet in DKD prevention.
In our study, subgroup analyses stratified by potential factors were established, followed by interaction terms to test the heterogenicity among different subgroups. The interaction between DII and stratified factors on DKD was not statistically significant, which ensures the reliability of the conclusion. This is in line with previous studies that identified similar associations between the Dietary Approaches to Stop Hypertension diet and kidney disease by sex, race and education level(Reference Rebholz, Crews and Grams33). In contract, a study identified the statistically significant interaction between BMI status and alternative healthy eating index on end-stage kidney disease risk(Reference Geng, Jafar and Neelakantan25). And, another study showed that the Dietary Approaches to Stop Hypertension diet was associated with rapid GFR decline among participants with hypertension but not among those without hypertension(Reference Liu, Kuczmarski and Miller34). Further research is necessary to replicate these findings from stratified analyses.
The cubic spline analysis further visualised the association between dietary inflammatory potential and DKD development. A cohort study of women aged 70 years indicated that there was a linear association between DII and the baseline renal function, renal function trajectory, suggesting that one-unit higher DII score was associated with a 0·55 ml/min per 1·73 m2 lower GFR at baseline and a 0·06 ml/min per 1·73 m2 greater annual decline in GFR over 10 years(Reference Bondonno, Blekkenhorst and Bird35). Another study conducted among older adults indicated that an increment of 1 sd in DII was associated with lower GFR, with a β of −1·8 % (95 % CI −2·7 %, −0·9 %)(Reference Xu, Sjögren and Ärnlöv18). Similarly, a linear dose–response pattern was found for the association between DII and DKD in the present study, in which 1-unit increasing of DII was associated with a 13 % higher odd of DKD. Previous studies have provided some supporting evidence for the benefits of maintaining an anti-inflammatory diet(Reference Ruiz-Canela, Zazpe and Shivappa36–Reference Hernáez, Castañer and Tresserra-Rimbau38). It is encouraging of the observed association that avoiding a pro-inflammatory diet could serve to be an additional, non-pharmacologic means for prevention of DKD. In future, more research is required to develop evidence-based preventive models, whether lowering intake of inflammation-promoting diet can translate to reducing the development of DKD.
Several possible mechanisms may explain the link between DII and DKD. First, a pro-inflammatory diet is positively associated with elevated inflammatory levels such as leucocyte counts(Reference Wirth, Sevoyan and Hofseth39). Although the metabolic disorder is historically considered as the pathogenesis of DKD, recent studies have established that the inflammatory responses also play a central role in progression of DKD. It has been shown that inflammation together with neutrophil–endothelium interactions could contribute to the pathogenesis of kidney injury, potentially leading to chronically impaired kidney function. Second, the dietary inflammatory potential is well recognised to regulate oxidative processes(Reference Man, Li and Xia40). It is reported that the oxidant–antioxidant imbalance plays an important pathogenic role in the development of diabetic complications, including diabetic nephropathy(Reference Flemming, Gallo and Forbes41,Reference Gyurászová, Gurecká and Bábíčková42) . Furthermore, the gut–kidney axis may represent a potential pathway underlying the inflammatory diet–DKD link(Reference Mosterd, Kanbay and van den Born7). Clinical trials have further shown the mechanistic involvement of gut microbiota in the pathophysiology of DKD by proving that gut microbiota can potentially trigger immune, metabolic and fibrotic pathways, which perpetuate the progression of renal pathology(Reference Chung, Barnes and Astroth43,Reference Nallu, Sharma and Ramezani44) . As has been reported that specific pro-inflammatory nutrients included in the calculation of DII serve to be key determinants of the modulating gut microbiota composition and activity(Reference Bolte, Vich Vila and Imhann45,Reference Mafra, Borges and Alvarenga46) .
One of the strengths is that this is the first study to explore the associations between the dietary inflammatory potential and DKD in a nationally representative sample of population. Second, data of NHANES are from a nationally representative sample of the USA, which enables the observed associations to be generalised. Third, the cubic spline analysis in our study, which characterises a dose–response association between a continuous exposure and an outcome, can help to clarify how the odd of DKD changes along with dietary inflammatory potential increasing. There are also some limitations in our study. First, our study is the cross-sectional design, which does not allow for inferences about causality. Second, as shown in online supplementary material, Supplemental Table 1, the proportion of excluded participants was relatively high, because of extreme energy intake, which might bring about bias in estimating the associations. Finally, the dietary data were from 24 h dietary recall interviews, and there might be ineluctable recall bias. Thus, well-designed longitudinal studies incorporating accurate assessment of measures are needed to fully clarify the causal relationship.
Our findings have public health and clinical significance, which is potentially important for not only the prevention but also the management of DKD. From the public health perspective, although this association was not causally shown, it may be legitimate to advise individuals to adhere to an anti-inflammatory diet to reduce their risk for kidney disease complications. Evidence-based public health education and publicity should be strengthened in an even broader segment of US population to raise awareness of altering dietary habits and promote an anti-inflammatory diet for DKD prevention. In addition, from the clinical perspective, future research should aim to evaluate the dietary inflammatory potential, develop nutritional protocol and consider incorporating it in dietary guidelines of managing DKD.
Conclusion
In conclusion, a more pro-inflammatory diet, as estimated by the higher DII score, was significantly associated with higher odd of DKD. Our findings emphasised the importance of developing novel nutritional approaches to prevent and manage DKD. Further clinical trials are required to strengthen the evidence of the associations between DII and DKD.
Acknowledgements
Acknowledgements: We gratefully acknowledge all of the study participants and staffs for their assistance during the study. Financial support: This work was supported by Shandong Provincial Key Research and Development Program (grant no. 2019GSF108196), Center of China–US Sports Economics and Health Engineering of Shandong (grant no. SDCA20191013), Academic Promotion Programme of Shandong First Medical University (grant no. 2019QL013) and Shandong Provincial Soft Science Research Program (grant no. 2020RKB14163). The funding sources had no role in study design, data analysis and interpretation of data, the writing of manuscript or the decision to submit the paper for publication. Authorship: X.L. and Z.C. conceived and designed the study; Y.W., Y.D. G.C. and Y.L. analysed the data; Y.W. and Y.L. wrote the paper; Y.L. reviewed the manuscript and had primary responsibility for the final content. All authors provided critical revisions of the manuscript and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the NCHS Research Ethics Review Board (ERB).
Conflicts of interest:
The authors declare no conflict of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980022001653