The broad field of nutrition and health is rife with myths, misconceptions and frequently posed yet seemingly fundamental questions that we intuitively feel should have simple answers. Is a calorie a calorie? Is obesity due to eating too much or doing too little? Is breakfast the most important meal of the day? Often there are simple answers, the first two being central to the themes considered in the present review and both absolutely ‘yes’ (just as a second is a second, one thermochemical calorie is simply a unit of measurement equivalent to 4·18 J). The third is not so easily answered and there can be no correct response until we refine that question; ‘If you wish to converse with me’ said Voltaire ‘define your terms’. In this case, we must define both what is meant by breakfast and what is meant by important (i.e. important for what?).
Framing our question in terms of whether breakfast is the most important meal of the day also implies some inherent value in comparing breakfast with other daily eating occasions. Why should the potential benefits of breakfast and therefore our decision about breakfast consumption depend on the relative importance of lunch or dinner? For example, breakfast consumption is unlikely to be more important for our general health than physical exercise or not smoking but that does not discount that breakfast may be sufficiently important to form part of a wider healthy lifestyle( Reference Cahill, Chiuve and Mekary 1 – Reference Odegaard, Jacobs and Steffen 4 ). Indeed, markers of a healthy lifestyle are associated with frequent breakfast consumption, which confounds interpretation of causal links between breakfast and good health.
The true question to be explored in the present review therefore concerns our daily decision about when to interrupt an extended period of fasting (e.g. overnight). Whether what might then be defined as breakfast and has the potential to cause meaningful effects on various health markers across different populations and contexts can then be considered. While this approach is unlikely to fit the false dichotomy through which the media obsessively brand any given health strategy as universally good or bad, the truth is understandably less extreme or consistent (i.e. breakfast is probably more or less important for some outcomes/people per day than for others).
What do we mean by ‘Breakfast’?
One issue contributing to the apparently conflicting findings in this area is that there is no universally accepted definition of breakfast( Reference O'Neil, Byrd-Bredbenner and Hayes 5 ); and why should there be? Without thinking about this too hard, it might at first seem logical simply to define breakfast as the first meal of the day. This is then consistent with the etymology to ‘break’ the ‘fast’ and may work for some as a general description of breakfast but is logically flawed and not overly helpful as a scientific definition. Consider an individual who breaks their fast shortly after waking by ingesting energy from carbohydrate, protein and fat in the form of coffee with milk and sugar, then nothing else until early-afternoon when the same mixed-macronutrients (plus alcohol) are consumed but this time in the form of spaghetti Bolognese and wine. Opinions may now be divided about whether this person had breakfast at all and, if so, whether it was coffee and/or spaghetti and wine. Can we count a cup of coffee as a meal? Was the spaghetti consumed in the fasted-state (i.e. post-absorptive)? What if we learn that this person woke at midday?
These differences of opinion become problematic when scientific investigations have surveyed breakfast habits or recommended breakfast consumption but allowed individual interpretation regarding what constitutes breakfast. This can be informative from a sociological perspective but it is helpful when considering physiological health effects to employ a more precise and consistent operational definition. Taking the earlier example, some studies have included only solid foods as breakfast irrespective of the many highly calorific beverages available, yet (notwithstanding differences in gastric emptying rate and metabolic response to different nutrients in solid v. liquid form( Reference Berry, Russo and Wishart 6 )), our net energy balance does not discriminate between absorbed nutrients or calories depending on whether they required chewing; ‘a calorie is a calorie’.
While in the future it might become possible to justify a rationale for defining meals based on a certain mixture of nutrients, a logical starting point to define the essential conditions of breakfast per se would be based on the quantity and timing of energy consumed. We propose that a quantity of 209·2 kJ (50 kcal) represents an appropriate arbitrary threshold to exclude common ingestive behaviours that would neither be recognised as a meal by the majority of people nor meaningfully shift our physiology towards the fed-state, a marker of which could be a detectable perturbation in exogenous and/or endogenous substrate utilisation (thus one standard tea/coffee would be unlikely to meet this criterion).
The issue of timing is more complex and can be considered relative to time of day, time of waking and/or the intervals that distinguish separate eating occasions. A universal definition of breakfast as morning feeding based purely on light–dark cycles (i.e. clock time) independent of sleep–wakes cycles (or vice versa) is complicated by variance in these very cycles due to geographical/seasonal differences in daylight hours or cultural/vocational differences in sleeping patterns (e.g. night-shift workers). A nominal period of 2 h after waking is also often applied to the definition of the breakfast meal, with separate meals in turn having been distinguished from snacks by a cut-off quantity of approximately 1087·8 kJ (260 kcal) and distinct eating occasions isolated on the basis of a 45 min interval( Reference de Castro 7 ). On balance, it therefore seems reasonable for a working definition of breakfast to represent the first meal consumed within 2 h after the longest sleep in any 24 h period, thus normally also reflecting the longest daily duration spent in the fasted-state and the only time most of us are genuinely post-absorptive( Reference Ruge, Hodson and Cheeseman 8 ).
According to the earlier rationale, our research involved approximately 70 lean and obese adults, of whom none worked night-shifts and approximately one-third habitually consumed <209·2 kJ (50 kcal) within 2 h of waking on most days, so might be classified as breakfast skippers. These individuals kindly participated in a series of experiments known as the Bath Breakfast Project, in which we allocated the habitual breakfast consumers and skippers equally into groups who for 6 weeks either: extended their overnight fast (0 kJ) until midday everyday; consumed 1464·4 kJ (350 kcal) within 2 h of waking and at least 2928·8 kJ (700 kcal) before 11.00 hours everyday; or maintained their usual lifestyles for 6 weeks( Reference Betts, Thompson and Richardson 9 ).
In contrast to the wealth of evidence contrasting different types or amounts of breakfast foods, this is the first randomised controlled trial to compare a treatment involving breakfast with the complete absence of morning feeding in relation to all components of energy balance. Whilst the project therefore ostensibly concerns breakfast (indeed, you may only be reading the present paper due to a shared interest in that meal), our intervention from a basic science perspective is in fact the fasting treatment, with morning feeding serving as a control (Bath Extended Morning Fasting Project did not seem so catchy). On that basis, the precise composition of breakfast prescribed was less important at this stage than simply ensuring that whatever was ingested differed sufficiently from fasting that meaningful effects would be detectable should they exist. The added practical benefits of this initial approach are that any significant effects could be generalised more broadly as responses to fasting as opposed to the presence or absence of specific foods consumed at breakfast; whereas none could argue that these treatments fail to polarise the contrast and meet all but the most extreme and unusual definitions of breakfast.
What do we mean by ‘Important’?
If you are hungry upon waking and personally prefer to promptly satiate your hunger, then breakfast is undoubtedly the most important (i.e. only) meal suited to that purpose. Similarly, if your morning will involve physical exercise with performance on that day a priority, then consuming a carbohydrate-rich breakfast is the most important meal to achieve your immediate goals( Reference Wright, Sherman and Dernbach 10 ). However, if we place importance on long-term health outcomes, these generally do not respond acutely to a single food or meal but instead require sustained exposure to a consistent dietary pattern. In this case, we are asking whether regular daily breakfast has a chronic effect on energy balance and associated health outcomes.
The present review will sequentially consider the effects of breakfast v. extended morning fasting on the various individual components of energy balance and health. For each outcome, we will first summarise the state of evidence linking breakfast to energy balance prior to our recent randomised controlled trial. That is the evidence upon which the pervasive societal beliefs about breakfast rested( Reference Brown, Bohan Brown and Allison 11 ), despite being almost entirely cross-sectional in nature. The vast and diverse populations surveyed are a legitimate strength of these epidemiological studies but are also responsible for misconceptions amongst a public (and media) ill-equipped to evaluate research design, measurement error or controls, so who are inclined only to believe the findings (or headlines) from studies perceived to be large (again, define your terms). Conversely, other studies are too often discounted for being small irrespective of accuracy and precision in measurement (for a primer see How big does my sample need to be?( Reference Batterham and Atkinson 12 )), which means we sometimes miss the opportunity to complement epidemiology with causal evidence from focused, tightly controlled and properly powered experiments (i.e. research where interventions and controls are directly manipulated). We will therefore set-out here how our understanding of causality specific to each outcome has been advanced by our recent series of randomised controlled trials; the Bath Breakfast Project.
Body mass/composition
As recently reviewed, although the extent to which the mere association between breakfast omission and obesity has been verified can be described as gratuitous, confirmatory studies continue to emerge even today despite the stated relationship confirmed by meta-analysis at a confidence level of P = 0·001 almost 20 years ago (rising to P < 10−42 at the most recent cut-off in 2011)( Reference Brown, Bohan Brown and Allison 11 ). There can be little doubt, therefore, that individuals who more frequently consume breakfast tend to be leaner and that this pattern hardly varies across a diverse range of human populations. However, no matter how strong these correlations may be, they cannot be used to draw a causal inference and so cannot inform evidence-based recommendations either encouraging or discouraging breakfast for the purposes of weight-management.
The Bath Breakfast Project was designed primarily to examine individual components of energy balance as opposed to long-term weight-change, as evident in the fact that the intervention was applied for only 6 weeks with direct prescription and adherence to the treatments (i.e. a completers-only analysis)( Reference Betts, Thompson and Richardson 9 ). In this sense, our examination of body mass changes as an indication of net energy (im)balance better reflects an efficacy trial and nicely complements the results of a concurrent effectiveness trial which reported no significant difference in weight-loss over 16 weeks with a recommendation to eat or skip breakfast (i.e. an intention-to-treat analysis)( Reference Dhurandhar, Dawson and Alcorn 13 ). Our data are consistent with this conclusion in that there was no significant difference in total body mass change between breakfast v. fasting amongst individuals who were either lean( Reference Betts, Richardson and Chowdhury 14 ) or obese( Reference Chowdhury, Richardson and Holman 15 ), although it is interesting to contrast the pattern of changes in dual-energy x-ray absorptiometry-derived body composition between groups across both levels of adiposity (Fig. 1).
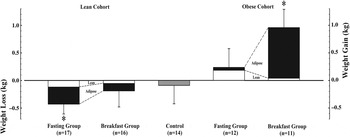
Fig. 1. Changes in dual-energy x-ray absorptiometry-derived body composition amongst lean( Reference Betts, Richardson and Chowdhury 14 ) and obese( Reference Chowdhury, Richardson and Holman 15 ) adults over 6 weeks with either ingestion of ≥2928·8 kJ (700 kcal) before 11.00 hours daily (Breakfast group), abstinence from all energy-providing nutrients until at least 12.00 hours daily (Fasting group) or lifestyle maintenance (Control). Data are means with se bars and * denotes a significant within group change from baseline (P < 0·05).
As can be seen, despite the absence of differences between groups according to the breakfast intervention, there were significant within-group changes from baseline but with the pattern reversed according to adiposity and treatment group. Specifically working from left to right across Fig. 1, lean individuals in the fasting group did not compensate for the energy ‘missed’ at breakfast, hence there is a significant reduction in body mass (mostly from fat loss); whereas lean individuals in the breakfast group certainly do not gain weight despite the relatively large prescription of at least 2928·8 kJ (700 kcal) by 11.00 hours daily for 6 weeks( Reference Betts, Richardson and Chowdhury 14 ). In contrast, it was the fasting group in the obese population who exhibited the greatest compensation, with avoidance of weight-loss despite consuming not a single calorie until midday every day for 6 weeks; whereas the obese individuals in the breakfast group clearly did not compensate by expending the prescribed energy intake (or reducing subsequent energy intake sufficiently) and so increased energy storage in the form of adipose tissue( Reference Chowdhury, Richardson and Holman 15 ).
The net effect of the earlier pattern is that, whether fed or fasted in the mornings, lean individuals may favour a more negative energy balance and obese individuals a more positive energy balance. This could mean that an individual's natural propensity to compensate is what determines the extent of adiposity and/or could equally mean that the extent of adiposity determines compensation. Whichever is the case, we begin to question both whether breakfast recommendations should vary according to adiposity and what mechanisms are involved in compensation (i.e. which components of energy balance are responsible)?
Components of energy balance
Energy intake
Cross-sectional observations
Omission of breakfast results in an energy intake deficit at the beginning of the day relative to breakfast consumption. Whether this deficit is maintained will depend on the existence/magnitude of compensatory feeding throughout the remainder of the day. Cross-sectional evidence predominantly suggests lower energy intake in those that skip breakfast( Reference Cho, Dietrich and Brown 16 – Reference Nicklas, O'Neil and Berenson 19 ), with a recent within person analysis from The National Health and Nutrition Examination Survey showing that energy intake is 1033·4 kJ (247 (95 % CI 121, 373) kcal) lower for men and 782·4 kJ (187 (95 % CI 121, 253) kcal) lower for women on days when breakfast was omitted (both P < 0·001)( Reference Kant and Graubard 20 ). However, this observation has not been consistent across all studies( Reference Mekary, Giovannucci and Willett 3 ), with work categorising individuals by graded breakfast frequency reporting no difference despite varying category definitions( Reference Mekary, Giovannucci and Cahill 2 , Reference Odegaard, Jacobs and Steffen 4 , Reference Wyatt, Grunwald and Mosca 21 ).
Acute laboratory studies
Experimental research has examined energy intake in both tightly-controlled acute settings in the laboratory and with chronic exposure to different morning feeding interventions under free-living conditions (i.e. people studied in their usual environment). The nature of laboratory investigations allows precise control and measurement of actual intake, yet it is that same tight control and elimination of external influences that presents a limitation when generalising to ‘real world’ behaviours( Reference Blundell, de Graaf and Hulshof 22 ). However, laboratory investigations allow measurement of other relevant variables such as concurrent metabolic measurements, subjective responses and appetite regulatory hormones, which can provide valuable mechanistic insight( Reference Karra and Batterham 23 ). The majority of appetite regulatory hormones previously measured are related to satiety and satiation (e.g. peptide tyrosine-tyrosine (PYY), glucagon-like peptide-1, leptin) but ghrelin acts as an appetite stimulant( Reference Cummings, Frayo and Marmonier 24 ). As would be expected, there are clear differences between morning fasting and breakfast consumption during the morning, with a postprandial reduction in ghrelin and increased PYY in response to breakfast consumption( Reference Chowdhury, Richardson and Tsintzas 25 , Reference Chowdhury, Richardson and Tsintzas 26 ), thus reflecting an anorexigenic response evidenced by subjective measures of appetite, as recently reviewed in this Journal( Reference Clayton and James 27 ).
Lunchtime feeding also elicits a PYY response that persists throughout the afternoon( Reference Chowdhury, Richardson and Tsintzas 25 , Reference Chowdhury, Richardson and Tsintzas 26 ), suggesting that this hormone reflects total cumulative intake as opposed to the energy content of the most recent meal. In contrast, both Clayton et al.( Reference Clayton, Barutcu and Machin 28 ) and our recent studies in lean( Reference Chowdhury, Richardson and Tsintzas 25 ) and obese( Reference Chowdhury, Richardson and Tsintzas 26 ) individuals suggest that, paradoxically, acylated ghrelin remains elevated during the afternoon in those that have consumed a carbohydrate-rich breakfast and lunch. This may be related to the reduced insulinaemic response to the lunchtime meal due to the second-meal effect( Reference Hamman and Hirschmann 29 ). While these findings for hormonal appetite regulatory mechanisms and results of subjective appetite assessments are informative, it is important to acknowledge that numerous factors contribute to appetite regulation( Reference Berthoud and Morrison 30 ). We have also shown in obese individuals that the pattern of appetite regulatory hormones and subjective appetite ratings does not necessarily predict ad libitum intake( Reference Chowdhury, Richardson and Tsintzas 26 ).
Studies investigating acute appetite regulation following breakfast omission fall into two main categories: those that have examined subsequent ad libitum energy intake following an unbroken overnight fast; and those where prior to lunch a pre-lunch snack (i.e. preload) was provided in both breakfast consumption/omission conditions such that lunch was always consumed in a fed state. In studies of lean individuals where lunch was consumed ad libitum, most but not all( Reference Levitsky and Pacanowski 31 , Reference Gonzalez, Veasey and Rumbold 32 ) indicate energy intake is increased at the lunch meal, both when fasted( Reference Chowdhury, Richardson and Tsintzas 25 , Reference Clayton, Barutcu and Machin 28 , Reference Levitsky and Pacanowski 31 ) and after a morning preload( Reference Astbury, Taylor and Macdonald 33 ). Of these studies, Astbury et al. report the energy deficit from breakfast was abolished by the increase in energy intake at lunch. This was not the case in our work in lean individuals( Reference Chowdhury, Richardson and Tsintzas 25 ), for whom total intake was greater in the breakfast condition. Notably the breakfast provided by Astbury et al. was relatively small (about 1046·0 kJ (250 kcal)) in comparison with those provided in most other investigations (typically >1673·6 kJ (400 kcal)). With this in mind, it is a logical suggestion that the energy content of larger breakfasts is less likely to be fully compensated in the next meal alone. Studies that have examined energy intake at both lunch and then dinner( Reference Clayton, Barutcu and Machin 28 ) or meals plus snacks( Reference Levitsky and Pacanowski 31 ) have not revealed increased intake after morning fasting, refuting the possibility that further compensation occurs throughout the day. This view is also supported by findings of similar energy intake during evening snacks and meals when comparing morning feeding v. fasting followed by a standardised lunch( Reference Thomas, Higgins and Bessesen 34 ).
The balance of evidence from controlled studies therefore suggests that breakfast omission results in some compensation at the next meal in lean individuals but that this next-meal effect is relatively transient with little evidence of more sustained compensatory feeding mechanisms. Interestingly, our work in obese individuals indicated similar energy intake at lunch independent of morning fasting or breakfast consumption( Reference Chowdhury, Richardson and Tsintzas 26 ). To our knowledge, this is the first report of ad libitum intake amongst obese adults after breakfast omission and subsequent investigations should attempt to determine if dietary compensation occurs at later feeding occasions in this population.
Intervention studies
Intervention studies attempting to quantify the response to chronic breakfast consumption or omission do not provide such clear evidence as laboratory investigations for the effect of breakfast omission upon energy intake. Early work in which feeding frequency was regimented throughout the day suggested that breakfast omission leads to greater energy intake than breakfast consumption( Reference Farshchi, Taylor and Macdonald 35 ). Two recent studies both from the same research group using similar cross-over designs of 1-week duration provide further data in this regard. In the first investigation, Halsey et al. ( Reference Halsey, Huber and Low 36 ) reported no difference in energy intake when participants either fasted or consumed an ad libitum high-carbohydrate breakfast under supervised laboratory conditions. In a subsequent investigation, participants were asked to consume a freely chosen breakfast within 1 h of waking for 1 week, relative to fasting until midday; omission of breakfast reduced daily energy intake by 669·4 kJ (160 kcal) relative to a mean energy intake of about 1673·6–2092·0 kJ (400–500 kcal) prior to midday when breakfast was consumed( Reference Reeves, Huber and Halsey 37 ).
Our recent investigations did not impose any dietary limitations on the participants in either group other than maintaining the morning fast until noon or consuming ≥2928·8 kJ (700 kcal) by 11.00 hours, with at least half of this consumed within 2 h of waking( Reference Betts, Thompson and Richardson 9 ). In lean individuals we found evidence for limited dietary compensation, with the breakfast group consuming 2255·1 kJ/d (539 (95 % CI 157, 920) kcal/d) more than those in the fasting group( Reference Betts, Richardson and Chowdhury 14 ). However, in the obese cohort energy intake was not significantly different between the breakfast and fasting groups, with those assigned breakfast intake consuming 1414·1 kJ/d (338 (95 % CI −313, 988) kcal/d) more( Reference Chowdhury, Richardson and Holman 15 ). This finding in obese individuals is consistent with the findings of Reeves et al.( Reference Reeves, Huber and Halsey 37 ), where the difference between breakfast and fasting groups was a pooled effect of lean (about 1108·7 kJ (265 kcal) higher) and obese individuals (about 251·04 kJ (60 kcal) higher), suggestive that obese individuals may compensate more for a morning energy deficit than lean individuals under free-living conditions. Interestingly, in our experiments the same obese individuals undertook both the acute investigation described earlier (where there was no compensation observed at lunch) and the free-living assessments (where there was no difference in daily intake between groups)( Reference Chowdhury, Richardson and Holman 15 , Reference Chowdhury, Richardson and Tsintzas 26 ). This is in contrast to the equivalent lean individuals who displayed limited compensation for breakfast omission both inside and outside the laboratory( Reference Betts, Richardson and Chowdhury 14 , Reference Chowdhury, Richardson and Tsintzas 25 ). The discord between these two groups of individuals suggests either that lean and obese people respond differently to the study designs employed or that energy intake may be more strongly influenced by environmental factors with increasing adiposity( Reference Mela 38 ). For example, the energy intake compensation evident in the obese cohort may be due to food choices and frequency, as opposed to the quantity consumed at single homogenous meals provided in an artificial laboratory setting.
As might be expected, the data from free-living investigations are inherently more varied than controlled laboratory investigations and the limitations of self-reported energy intake have recently been detailed elsewhere( Reference Dhurandhar, Schoeller and Brown 39 ). While these factors contribute towards systematic and random error and so impact both validity and reliability, there is little reason to believe that comparisons between experimental groups would be systematically biased by such limitations( Reference de Castro 7 ). Nonetheless, methods to assess diet remain challenging under free-living conditions and there are currently no viable alternatives to dietary records in some form if specific nutrient profiles and/or feeding patterns are of interest. However, from a pure energy-balance perspective, it is possible to estimate total energy intake with relative accuracy using the intake-balance method( Reference Gilmore, Ravussin and Bray 40 , Reference de Jonge, DeLany and Nguyen 41 ), which exploits the energy-balance equation to derive energy entering the system as the sum of the change in energy storage and objectively measured energy expenditure( Reference Racette, Das and Bhapkar 42 ). The latter may itself be responsive to altered feeding patterns and the following sections will address this possibility with specific reference to each individual component of energy expenditure.
Resting metabolic rate
RMR is for a large proportion of individuals the greatest contributor to energy expenditure( Reference Carpenter, Poehlman and O'Connell 43 ). Decreases in mass adjusted RMR have been demonstrated in both starvation and hypoenergetic dieting( Reference Doucet, St-Pierre and Almeras 44 – Reference Martin, Heilbronn and de Jonge 46 ) but evidence for a modifying effect of chronic morning feeding pattern upon RMR is not apparent. Three past studies have measured changes in RMR in response to a sustained morning feeding intervention( Reference Farshchi, Taylor and Macdonald 35 , Reference Schlundt, Hill and Sbrocco 47 , Reference Reeves, Huber and Halsey 48 ). Of these, Schlundt et al. ( Reference Schlundt, Hill and Sbrocco 47 ) demonstrated that weight loss induced by caloric restriction in obese women resulted in similar reductions in RMR whether consuming breakfast or fasting during the morning. In accordance, the 2-week crossover intervention of Farshchi et al. found no difference in RMR (or weight/body composition) following breakfast consumption or skipping regimens in lean women( Reference Farshchi, Taylor and Macdonald 35 ). In a crossover study design involving groups of lean and overweight individuals, 1 week of breakfast consumption or fasting until noon also had no effect upon RMR( Reference Reeves, Huber and Halsey 48 ).
The results of our 6-week interventions in both lean( Reference Betts, Richardson and Chowdhury 14 ) and obese( Reference Chowdhury, Richardson and Holman 15 ) individuals over 6 weeks of daily breakfast or morning fasting indicated that RMR was unaffected by morning feeding pattern (all groups stable within 62·8 kJ/d (15 kcal/d)). Therefore, the evidence uniformly shows that consistently extending the overnight fast does not directly affect RMR beyond the predicted change associated with possible changes in body mass/composition.
Diet-induced thermogenesis
Diet-induced thermogenesis (DIT) is the smallest component of energy expenditure under most circumstances and reflects the obligatory energy expended for the processing and digestion of food. Different macronutrients induce varying levels of thermogenesis( Reference Tappy 49 , Reference Westerterp, Wilson and Rolland 50 ), but DIT is only ever a fraction of the energy content of the foods ingested and typically only about 10 % of intake when consuming a normal mixed diet( Reference Westerterp 51 ). Only one intervention study has examined the effect of a sustained morning feeding intervention on DIT, with no effect on the thermic effect of a mixed macronutrient test drink after breakfast skipping or consumption for 2 weeks( Reference Farshchi, Taylor and Macdonald 35 ).
There is some evidence that DIT is greater in the morning than later in the day( Reference Bo, Fadda and Castiglione 52 , Reference Romon, Edme and Boulenguez 53 ) and the thermogenic effect of breakfast is necessarily greater than morning fasting. Indeed, when consuming breakfast and an ad libitum lunch, both lean and obese participants expend greater energy through DIT during the morning and afternoon than when omitting breakfast (276·1 (sd 138·1) kJ (66 (sd33) kcal) v. 205·0 (sd 121·3) kJ (49 (sd 29) kcal) in lean and 284·5 (sd 125·5) kJ (68 (sd 33) kcal) v. 167·4 (sd 96·2) kJ (40 (sd 23) kcal) in obese; Chowdhury et al., unpublished results). In studies where a fixed lunch meal has been provided following morning fasting/feeding, DIT during the afternoon was greater following breakfast( Reference Thomas, Higgins and Bessesen 34 ) or not different relative to fasting when measured 1 and 4 h after lunch( Reference Clayton, Barutcu and Machin 28 ). Where energy intake has been matched across 24 h following breakfast omission by increasing intake at subsequent meals, no difference in 24 h energy expenditure was observed( Reference Kobayashi, Ogata and Omi 54 ). This suggests little modifying effect of morning feeding pattern on DIT. Future studies should determine the effect of chronic breakfast omission upon DIT in response to feeding (i.e. a chronic adaptation in the acute response). However, any potential effect of breakfast consumption per se on overall DIT will be quantitatively small and inexorably outweighed by the energy intake required to elicit that DIT.
Physical activity thermogenesis
Of the components contributing to total energy expenditure, physical activity thermogenesis is undoubtedly the most modifiable component yet has received surprisingly little attention in the literature regarding breakfast. Higher physical activity levels assessed by questionnaire are cross-sectionally associated with regular breakfast consumption( Reference Cahill, Chiuve and Mekary 1 – Reference Mekary, Giovannucci and Willett 3 , Reference Wyatt, Grunwald and Mosca 21 , Reference Smith, McNaughton and Cleland 55 – Reference Barr, DiFrancesco and Fulgoni 57 ). However, this relationship has not been explained by causal data from experimental studies, with the few that are available having employed a wide variety of methodologies of varied sensitivity and specificity. Several studies have investigated the effect of varying feeding frequencies upon overall energy expenditure measured using a whole body calorimeter( Reference Smeets and Westerterp-Plantenga 58 – Reference Taylor and Garrow 60 ), which understandably places severe restrictions upon natural physical activity patterns that might be responsive to breakfast outside the laboratory.
Other past studies have attempted to quantify aspects of physical activity behaviour in response to breakfast in particular or altered daily meal frequency in general using a variety of approaches. Physical movements have been estimated using hip-worn monitors, pedometers or accelerometers but have failed to detect any difference in step counts during 1 week of either breakfast or fasting( Reference Halsey, Huber and Low 36 , Reference Reeves, Huber and Halsey 48 ) or any difference in accelerometer counts when comparing a three-meal feeding pattern with a single evening-meal for 8 weeks( Reference Stote, Baer and Spears 61 ). However, natural adjustments in overall activity may have been masked in the latter study because participants were ‘encouraged to maintain their normal exercise throughout the day’. In addition, such measurement tools may also lack both reliability and sensitivity when applied to subtle changes across all aspects of physical activity thermogenesis( Reference Thompson, Batterham and Bock 62 ). While the issues of reliability and sensitivity have been overcome using doubly-labelled water to verify no difference in total energy expenditure between a two- v. seven-meal daily feeding pattern( Reference Verboeket-van de Venne, Westerterp and Kester 63 ), that finding is neither specific to breakfast or physical activity thermogenesis per se, nor does the technique reveal temporal patterns of activity.
We employed combined heart-rate accelerometry as a validated tool to quantify physical activity thermogenesis on a minute-by-minute basis under free-living conditions in response to our daily breakfast v. fasting intervention. This instrument is particularly sensitive to the low-to-moderate intensity, spontaneous lifestyle activities that we hypothesised might be most responsive to breakfast( Reference Betts, Thompson and Richardson 9 , Reference Thompson, Batterham and Bock 62 ). Our investigation in lean individuals demonstrated that daily physical activity thermogenesis was substantially greater amongst those consuming breakfast than those fasting (1849·3 (95% CI 142·3, 3560·6) kJ/d (442 (95% CI 34, 851) kcal/d)), with a particular difference between groups apparent for the morning period and for light intensity activities( Reference Betts, Richardson and Chowdhury 14 ). The obese individuals subsequently studied were less active overall and did not display such a difference between groups in total daily physical activity thermogenesis (1138·0 (95% CI 1309·6, 4133·8) kJ/d (272 (95% CI −313, 988) kcal/d)) although, like their lean counterparts, an effect on morning energy expenditure was apparent (786·6 (95% CI 167·4, 1401·6) kJ/d (188 (95% CI 40, 335) kcal/d))( Reference Chowdhury, Richardson and Holman 15 ). This suggests that modifying feeding patterns can affect physical activity, with the most pronounced response during the time period of energy restriction/breakfast consumption. The reasons for this are not immediately clear but might be related to perceptions of lethargy, expectations relating to physical activity readiness or that reduced availability of exogenous substrate and/or systemic metabolites may limit engagement in non-essential physical exertion.
Taken collectively, these observations that physical activity levels are lower in response to fasting begin to explain why a resolution to start skipping breakfast may not predict the degree of weight loss one might expect. The shaping of our genome prior to the agricultural revolution ensured that individuals evolved mechanisms to protect against energy deficit during natural fed–fasted cycles on a daily basis (i.e. when almost every meal required initial ‘investment’ of energy). In this sense, it might be better to express the energy-balance equation not as Balance = Intake − Expenditure but instead Balance = −Expenditure + Intake. The net result is unchanged but this serves as a reminder that, in terms of survival, our investment of energy comes first and is inevitable, whereas food availability/procurement is uncertain and may be zero.
Strategies designed to improve human health by targeting energy balance must therefore integrate an appreciation of how compensatory feedback mechanisms can operate to defend against energy deficit. Conserving energy via reduced physical activity can be effective in the short term, but may not favour survival during a sustained food shortage, in which case more sedentary behaviours might be selected-out relative to the more proactive approach of competing for what limited resources are available early in the post-absorptive period. It therefore remains a possibility that more extreme or sustained exposure to extended daily fasting resulting in a chronically hypoenergetic diet could stimulate increased spontaneous physical activities, similar to the starvation-induced hyperactivity noted in rodents and patients with anorexia( Reference Hebebrand, Exner and Hebebrand 64 ). Of course, these elegantly evolved compensatory mechanisms have become somewhat obsolete (for most) in modern societies where food procurement is largely independent of any up-front investment of energy( Reference Eaton and Eaton 65 ). An effective intervention today will therefore need to target both sides of the energy-balance equation (e.g. diet and physical activity); hence, the following section will consider the arguably more natural scenario in which fasting is superimposed against a background of physical activity and/or exercise.
Exercise–fasting interactions
An important distinction should be made between physical activity thermogenesis and exercise-induced thermogenesis. Whilst both have an end result of increasing energy expenditure, the distinguishing factor is that the latter is defined by having a purpose. Accordingly, if structured exercise was already planned for as part of an individual's morning, then this is likely to prohibit the effect of breakfast consumption on physical activity thermogenesis, since energy expenditure is prescribed. The question then arises, what are the effects of breakfast consumption on metabolism for the morning exerciser?
The acute responses of exercise metabolism to prior feeding are well characterised. Total energy expenditure is almost entirely determined by the duration and intensity of the exercise bout, but substrate selection can be drastically shifted by nutritional status. Consumption of a mixed-macronutrient breakfast increases carbohydrate oxidation and suppresses fat oxidation during exercise( Reference Gonzalez, Veasey and Rumbold 32 , Reference Wu and Williams 66 ), which is largely driven by the type and quantity of carbohydrate in the meal( Reference Wu, Nicholas and Williams 67 ). This is predominantly due to the insulin-induced suppression of plasma NEFA availability; insulin concentrations after a mixed-macronutrient carbohydrate-rich breakfast remain elevated sufficient to all but maximally suppress palmitate appearance( Reference Jensen, Caruso and Heiling 68 ). Interestingly, the breakfast-induced suppression of fatty acid availability during exercise is not due to a reduction in lipolysis (at least in the subcutaneous adipose tissue depot) but rather to an increase in re-esterification( Reference Enevoldsen, Simonsen and Macdonald 69 ). In addition, if the breakfast has a particularly high glycaemic index, then an elevated pre-exercise muscle glycogen concentration( Reference Wee, Williams and Tsintzas 70 ) can also contribute to a further suppression of fat oxidation in both men( Reference Wee, Williams and Gray 71 ) and women( Reference Stevenson, Williams and Nute 72 ).
The omission of breakfast prior to exercise (or delaying breakfast consumption until after exercise) also appears to have unique consequences for acute whole-body substrate balance. Physical exercise does not invoke the same acute energy intake response to breakfast omission/delay presented earlier (i.e. energy intake at lunch and dinner is largely either unaltered( Reference Gonzalez, Veasey and Rumbold 32 , Reference Deighton, Zahra and Stensel 73 , Reference Farah and Gill 74 ) or does not fully compensate for breakfast omission( Reference Clayton, Barutcu and Machin 28 )). Instead, the increase in energy expenditure due to exercise, combined with the shift in substrate utilisation towards greater lipid oxidation with breakfast omission, results in a less positive (more negative) fat balance in both lean( Reference Gonzalez, Veasey and Rumbold 32 ) and overweight men( Reference Farah and Gill 74 ). This has also been observed over a full 24-h period with room calorimetry and fixed energy intake( Reference Shimada, Yamamoto and Iwayama 75 ). Given the importance of endogenous carbohydrate stores for exercise tolerance( Reference Bergstrom, Hermansen and Hultman 76 – Reference Casey, Short and Hultman 78 ), the preservation of whole-body carbohydrate balance in the presence of a negative fat balance( Reference Gonzalez, Veasey and Rumbold 32 , Reference Farah and Gill 74 ) could be an attractive metabolic milieu for the regular exerciser.
The chronic effect of breakfast–exercise interactions is much less clear. An emerging theme in exercise physiology is the augmentation of endurance-type training adaptations through manipulation of substrate availability. Methods such as multiple bouts of exercise( Reference Hansen, Fischer and Plomgaard 79 , Reference Yeo, McGee and Carey 80 ), reductions in dietary carbohydrate intake and timing of dietary carbohydrate intake( Reference Marquet, Brisswalter and Louis 81 , Reference Van Proeyen, Szlufcik and Nielens 82 ) all serve to reduce endogenous or exogenous carbohydrate availability, consequently elevating fatty acid availability. Whilst (to the authors knowledge) no studies are available on the effect of breakfast on endurance training adaptation per se, there is evidence to suggest that consumption of a carbohydrate-rich breakfast prior to training, in addition to carbohydrate intake during every exercise training session can impair some endurance-type training adaptations. Specifically, compared to extending the overnight fast until after exercise, carbohydrate consumption before and during exercise can attenuate and/or abolish the increases in VO2max ( Reference Van Proeyen, Szlufcik and Nielens 83 ) glucose tolerance, insulin sensitivity, resting muscle glycogen concentrations and GLUT4 content( Reference Van Proeyen, Szlufcik and Nielens 84 ). It should be noted however, that these effects are not consistent across all studies of fasted-state exercise training( Reference De Bock, Derave and Eijnde 85 ).
The energy balance and body composition responses to regular exercise training with breakfast consumption/omission are currently unknown. It therefore remains to be seen whether the Nobel Laureate and Exercise Physiologist A.V. Hill had a firm rationale for running a mile every morning prior to having breakfast( Reference Hill and Lupton 86 ).
Health outcomes
Much of the work examining different morning feeding patterns as described in the present review has focused on components of energy balance. Considering the severity of the growing issue of obesity( Reference Wang, McPherson and Marsh 87 ) and the general preoccupation of the public/media with the effects of diet upon weight, this is not surprising. However, it is important to keep in mind that the primary reason for the study of energy balance is not as an endpoint in itself, but because of our interest in the potential impact of an individual's energy (im)balance upon factors that may then affect their health. While chronic energy (im)balance is potentially an important contributor to negative health outcomes, specific components of energy balance such as physical activity can also impact disease and mortality risk independent of net energy surplus/deficit or changes in adiposity( Reference Ekelund, Ward and Norat 88 , Reference Walhin, Richardson and Betts 89 ). Therefore, it is perfectly plausible that the omission/consumption of breakfast might affect markers of health independent of energy balance.
While there is a wealth of evidence for increased disease risk in those that omit breakfast( Reference Cahill, Chiuve and Mekary 1 – Reference Odegaard, Jacobs and Steffen 4 ), randomised controlled trials that have provided causal mechanisms to explain these observations remain very limited. In the two prior studies where health markers have been measured, Stote et al. ( Reference Stote, Baer and Spears 61 ) report increased lipoproteins relative to a three-meal pattern (total, HDL and LDL) when individuals adhered to a one-meal a day regimen. In a less extreme model, Farshchi et al.( Reference Farshchi, Taylor and Macdonald 35 ) report when delaying morning intake until 10.30 hours each morning for 2 weeks that total and LDL-cholesterol and insulin response to a test drink increased (although other measures of insulin sensitivity remained unchanged), relative to a reduction when consuming breakfast daily. Our recent studies have extended this evidence by measuring several markers related to CVD risk and metabolic control. In lean individuals, only a modest increase in glucose variability in those fasting during the afternoon/evening was detected( Reference Betts, Richardson and Chowdhury 14 ), with no effects for 24 h glycaemic control detected in obese individuals( Reference Chowdhury, Richardson and Holman 15 ). However, there was an interaction effect for insulinaemic response to an oral glucose tolerance test in this population, with a reduction in those consuming breakfast relative to an increase in those fasting. Across both groups, the majority of health markers were unaffected by either regimen. Therefore, it appears that any effects of chronic morning fasting upon health in healthy individuals are either non-existent or not detectable over the relatively short time period examined. Evidence for a potential effect upon insulin sensitivity and glycaemic control is evident in the work of our group and others( Reference Betts, Richardson and Chowdhury 14 , Reference Chowdhury, Richardson and Holman 15 , Reference Farshchi, Taylor and Macdonald 35 ), and tallies somewhat with reports of improved glycaemic control with greater breakfast quantity in type-2 diabetics( Reference Jakubowicz, Wainstein and Ahren 90 , Reference Rabinovitz, Boaz and Ganz 91 ). However, considering that not all measures of metabolic control demonstrated a deterioration with extended morning fasting in healthy individuals, it appears that any effects are subtle at best. Future studies could provide further insight by employing interventions of longer durations, over which potential effects upon markers of health might be more apparent.
Conclusions
The evidence reviewed suggests that breakfast omission affects some components of energy balance much more than others. There is no evidence to suggest that breakfast consumption per se affects RMR, or DIT of subsequent meals or over the day as a whole. Evidence that breakfast affects energy intake is compelling for laboratory studies, with the majority of studies showing energetic compensation at the next meal, but not sufficient to eliminate the deficit from morning fasting. In addition, designs where afternoon/evening feeding has been allowed do not demonstrate sustained compensation for breakfast omission. Experiments outside the laboratory understandably produce more varied results, with the balance of evidence suggesting that energy intake is either lower or similar when omitting breakfast. Our work in lean and obese groups would suggest that there are differences between groups in energy intake responses based on adiposity. The body of evidence taken together supports the concept that, in general, energy intake is reduced when breakfast is omitted, with limited support for the popular perception of greater overall energy intake after breakfast omission.
While much work has investigated energy intake in response to breakfast omission, there is a severe lack of studies investigating the most modifiable component of energy expenditure-physical activity energy expenditure, with some studies limited by measurement issues. Our work in both lean and obese individuals suggests that breakfast omission may lower physical activity energy expenditure, particularly during the morning, although this needs confirmation and the potential reasons for this phenomenon remain to be established. The majority of studies conducted to date have been of relatively short duration, but those that have examined the effect of breakfast omission upon body weight do not support the strongly established public perceptions and correlational evidence that omission of breakfast is associated with weight-gain.
Future investigations should focus on concurrently measuring all aspects of energy balance, to provide a fuller understanding of the effects of breakfast omission upon individual components (and importantly the interaction of these components). Longer-term studies are needed to conclusively establish the effects of breakfast omission upon health markers, with more studies required examining overweight and obese populations. Breakfast may or may not be the most important meal of the day, but it is certainly an important meal to investigate further.
Acknowledgements
The authors thank those who participated in the trial for their time and commitment.
Financial Support
This research was funded by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC; BB/H008322/1) and is registered at www.isrctn.org (ISRCTN31521726).
Conflicts of Interest
None.
Authorship
J. A. B. has provided consultancy for PepsiCo, Lucozade Ribena Suntory and Kellogg, J. T. G. has provided consultancy for PepsiCo, Lucozade Ribena Suntory and FrieslandCampina. J. A. B., K. T. and D. T. designed the research; J. A. B., J. D. R., E. A. C. and D. T. conducted the research; K. T. provided essential reagents and materials; J. A. B., E. A. C. and J. D. R. analysed the data and performed statistical analysis; E. A. C., J. A. B. and J. T. G. co-wrote the paper and have primary responsibility for final content. All authors read, edited and approved of the final manuscript.






