Article contents
A historical and systematic overview of Ascaris vaccine development
Published online by Cambridge University Press: 09 August 2021
Abstract
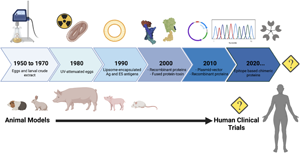
Ascariasis is the most prevalent helminth infection in the world and leads to significant, life-long morbidity, particularly in young children. Current efforts to control and eradicate ascariasis in endemic regions have been met with significant challenges including high-rates of re-infection and potential development of anthelminthic drug resistance. Vaccines against ascariasis are a key tool that could break the transmission cycle and lead to disease eradication globally. Evolution of the Ascaris vaccine pipeline has progressed, however no vaccine product has been brought to human clinical trials to date. Advancement in recombinant protein technology may provide the first step in generating an Ascaris vaccine as well as a pan-helminthic vaccine ready for human trials. However, several roadblocks remain and investment in new technologies will be important to develop a successful human Ascaris vaccine that is critically needed to prevent significant morbidity in Ascaris-endemic regions around the world.
- Type
- Review Article
- Information
- Parasitology , Volume 148 , Special Issue 14: Lessons from studying roundworm and whipworm in the mouse - common themes and unique features , December 2021 , pp. 1795 - 1805
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 6
- Cited by





