Article contents
Exploring the role of macrophages in determining the pathogenesis of liver fluke infection
Published online by Cambridge University Press: 27 May 2022
Abstract
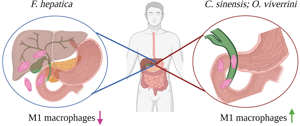
The food-borne trematodes, Opisthorchis viverrini and Clonorchis sinensis, are classified as group 1 biological carcinogens: definitive causes of cancer. By contrast, infections with Fasciola hepatica, also a food-borne trematode of the phylum Platyhelminthes, are not carcinogenic. This review explores the premise that the differential activation of macrophages during infection with these food-borne trematodes is a major determinant of the pathological outcome of infection. Like most helminths, the latter stages of infection with all 3 flukes induce M2 macrophages, a phenotype that mediates the functional repair of tissue damaged by the feeding and migratory activities of the parasites. However, there is a critical difference in how the development of pro-inflammatory M1 macrophages is regulated during infection with these parasites. While the activation of the M1 macrophage phenotype is largely suppressed during the early stages of infection with F. hepatica, M1 macrophages predominate in the bile ducts following infection with O. viverrini and C. sinensis. The anti-microbial factors released by M1 macrophages create an environment conducive to mutagenesis, and hence the initiation of tumour formation. Subsequently, the tissue remodelling processes induced by the M2 macrophages promote the proliferation of mutated cells, and the expansion of cancerous tissue. This review will also explore the interactions between macrophages and parasite-derived signals, and their contributions to the stark differences in the innate immune responses to infection with these parasites.
Keywords
- Type
- Review Article
- Information
- Parasitology , Volume 149 , Special Issue 10: Foodborne Trematodes – time to rise from neglected status , September 2022 , pp. 1364 - 1373
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 6
- Cited by





