Article contents
Olive oil and wine as source of multi-target agents in the prevention of Alzheimer disease
Published online by Cambridge University Press: 13 December 2021
Abstract
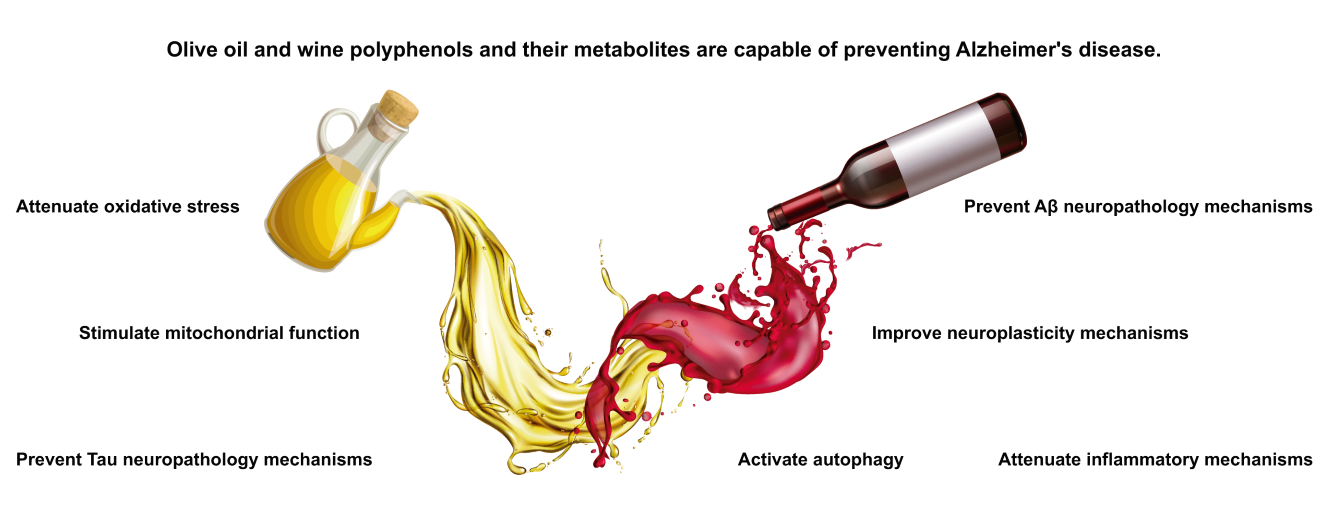
Olive oil and wine are consumed daily worldwide, and they constitute the fundamental pillars of the healthy Mediterranean diet. Polyphenolic compounds, naturally present in both olive oil and wine, are responsible for their beneficial properties. Current studies have shown the neuroprotective effects of polyphenols independently of their well-known antioxidant action. In this work, we have focused on reviewing the protective effect of polyphenols from extra virgin olive oil and wine in Alzheimer´s disease (AD), to emphasise that both foods could be a possible therapeutic tool. Beneficial effects have been described in β-aggregation, neurofibrillary tangles, autophagy and mitochondrial function, as well as in cerebral insulin resistance. Furthermore, to date, a harmful dose has not been described. Both pre-clinical and clinical works demonstrate that polyphenols act on neuropathological and cognitive disorders of AD, preventing or stopping the onset of this devastating disease. However, there are certain limitations in these studies, since it is very difficult to research diseases that lead to cognitive impairment. Although all the findings obtained are very encouraging, more studies should be carried out investigating the use of the polyphenols from olive oil and wine as therapeutic agents in the progression of AD. Therefore, more longitudinal studies in humans with a homogeneous cohort of patients are necessary to corroborate the efficacy of these nutraceuticals, as well as determine the most appropriate dose for this purpose.
Keywords
- Type
- Review Article
- Information
- Copyright
- © The Author(s), 2021. Published by Cambridge University Press on behalf of The Nutrition Society
References
- 5
- Cited by





