Introduction
Cultivation of cereal crops and consumption of grains and cereal products have made gluten-containing grains a great part of human evolution and food habits since agricultural development began about 9000 years BC( Reference Freeman 1 ). Since the beginning of the agricultural revolution, the production and utilisation of wheat have gradually expanded globally, and today wheat-containing foods provide as much as up to 50 % of energy intake in both industrialised and developing countries, making it a vital part of the population’s diet( Reference Tovoli, Masi and Guidetti 2 ).
Wheat contains carbohydrates (60–65 %), lipids (1–2 %), proteins (10–14 %) and water. Gluten is made up of a complex mixture of hundreds of different proteins that are collectively named gluten. The mixture is divided into the different protein complexes gliadin and glutenin. Gliadin is an alcohol-soluble fraction further divided into α-, β-, γ- and ω-gliadins; glutenin, which is an alcohol-insoluble substance, is further divided into high-molecular-weight and low-molecular-weight glutenins( Reference Lundin, Qiao and Snir 3 , Reference Ludvigsson, Leffler and Bai 4 ). The protein structure of wheat gluten is very similar to the proteins of rye (secalin) and barley (hordein) and, therefore, the term gluten is often used as a collective term to describe all of these components. Wheat also contains non-gluten proteins, such as amylase–trypsin inhibitors (ATI)( Reference Lundin, Qiao and Snir 3 ).
During the last decade, the avoidance of wheat and other gluten-like-containing grains has increased worldwide, with the highest prevalence in the Western countries presumed to be 10–20 %( Reference Foschia, Horstmann and Arendt 5 , Reference Makharia, Catassi and Makharia 6 ). This decline in wheat intake is due to the fact that the ingestion of gluten has been putatively linked to a wide range of health complaints and clinical disorders, such as gastrointestinal symptoms including abdominal pain, skin lesions, migraines, weight gain, fatigue, depression and tiredness( Reference Biesiekierski, Muir and Gibson 7 ).
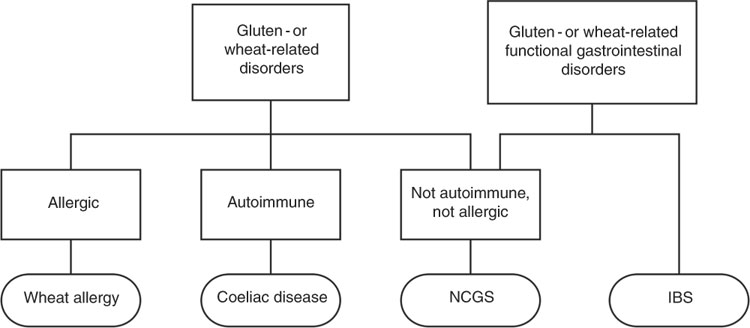
The spectrum of gluten-related disorders includes coeliac disease (CD), wheat allergy (WA) and the new entity non-coeliac gluten sensitivity (NCGS). The estimated global prevalence of these gluten-related disorders is about 5 %; however, the prevalence of individuals following a gluten-free diet (GFD) is much higher( Reference Elli, Branchi and Tomba 8 ). The diagnosis of both CD and WA is relatively straightforward due to the presence of objective examinations and biomarkers. On the other hand, NCGS remains a diagnosis based on the exclusion of other diagnoses with lack of biomarkers and no standardised procedure. It remains to be clarified whether the condition is linked to gluten alone, or whether substances other than gluten may be involved in the symptom generation( Reference Catassi, Elli and Bonaz 9 ).
The present review aims to give an overview of the different gluten-related disorders, focusing especially on the factors other than gluten assumed to have a role in the onset of NCGS.
Gluten-related disorders
Coeliac disease
CD is defined as ‘a chronic small intestinal immune-mediated enteropathy, precipitated by exposure to dietary gluten in genetically predisposed individuals’( Reference Ludvigsson, Leffler and Bai 4 ). The disease is characterised as an immunological response to ingested gluten, affecting only individuals who are genetically disposed. Of the population, 30–40 % carry the human leucocyte antigen (HLA) DQ2 and/or DQ8 genotypes, predisposing for CD and seen in 95 % of all CD patients( Reference Catassi, Bai and Bonaz 10 ).
Gluten is poorly digested in individuals both with and without CD. When an individual with CD ingests gluten, intact gluten peptides cross into the submucosa of the small intestine, where the human enzyme transglutaminase 2, also known as tissue transglutaminase (tTG), deaminates gluten peptides. The deaminated gluten peptides make high-affinity bindings to HLA-DQ2/8 molecules( Reference Ludvigsson, Leffler and Bai 4 ). Intestinal T-lymphocytes are activated via the T-cell receptor by the interaction of HLA-DQ2/8 molecules on antigen-presenting cells. Pro-inflammatory cytokines such as interferon-γ, TNF-α and IL-2 are released from the activated T-cell. These cytokines damage the enterocytes and produce the intestinal lesions typical for CD( Reference Mocan and Dumitrascu 11 ).
The global prevalence of CD is approximately 1 %( Reference Ludvigsson, Bai and Biagi 12 ). A higher prevalence is seen in Northern European countries, with a prevalence of approximately 1·5 % in the Northern countries. The highest prevalence in the world has been described in an African population living in Western Sahara, with an occurrence of 5·6 %( Reference Aziz, Dwivedi and Sanders 13 ). The diagnosis is given to patients of all ages, and about 20 % receive the diagnosis after reaching 60 years old. CD was previously seen as a childhood disease, with typical symptoms of weight loss, diarrhoea, malabsorption and growth retardations, but this clinical picture is relatively rare today, due to better awareness and understanding of its presentation allowing earlier detection and diagnosis. CD is more prevalent in patients with type 1 diabetes, thyroiditis, inflammatory bowel disease, Addison’s disease and systemic lupus erythematosus, than in the rest of the population( Reference Aziz, Dwivedi and Sanders 13 ).
The classical symptoms of CD include gastrointestinal symptoms such as diarrhoea, steatorrhea, abdominal pain and growth retardation or weight loss due to malabsorption( Reference Ludvigsson, Leffler and Bai 4 , Reference Ludvigsson, Bai and Biagi 12 ). Nearly half of CD patients also experience atypical symptoms such as fatigue, anaemia, osteoporosis, dermatitis herpetiformis, neurological issues, depression, infertility and dental enamel hypoplasia. The most common form of CD can be diagnosed during all stages of life, and is characterised by malabsorption, crypt hyperplasia and villi atrophy. However, the symptoms can occur in different combinations and severity, and some patients diagnosed with CD experience no symptoms at all, referred to as silent CD( Reference Ludvigsson, Leffler and Bai 4 , Reference Mocan and Dumitrascu 11 , Reference Aziz, Dwivedi and Sanders 13 ).
For a correct diagnosis of CD, the patient must be on a gluten-containing diet. The serological characteristics of CD involve the presence of specific endomysial antibodies, anti-tissue transglutaminase antibodies (α-tTG) and/or deaminated gliadin peptide antibodies( Reference Ludvigsson, Leffler and Bai 4 , Reference Thomas and Bishop 14 ). The presence of these antibodies in the blood strongly supports the diagnosis, but they need to be followed by a duodenal biopsy (via gastroscopy) to confirm the diagnosis( Reference Ludvigsson, Leffler and Bai 4 , Reference Ludvigsson, Bai and Biagi 12 ).
The only recommended treatment for CD is to follow a strict, lifelong GFD, which implies a diet free of wheat, rye and barley( Reference Ludvigsson, Leffler and Bai 4 , Reference Aziz, Dwivedi and Sanders 13 , Reference Silvester, Graff and Rigaux 15 ). It is extremely important for patients with CD to be adherent to the diet to achieve full recovery of the bowel( Reference Biagi, Andrealli and Bianchi 16 ). Failure to follow a strict GFD may lead to long-term complications, including malabsorption and nutrient deficiencies, peripheral neuropathy, infertility, osteoporosis and increased risk of lymphoma, resulting in overall increased mortality( Reference Freeman 1 ) Different drugs that may allow gluten-intolerant patients to consume some small doses of gluten, without the risk of causing mucosal damage, are currently being trialled (for example, Larazotide)( Reference Freeman 1 , Reference Kelly, Green and Murray 17 – Reference Leffler, Kelly and Abdallah 19 ). It is anticipated that these initial drugs may be on the market during 2018; however, further studies are needed investigating their effectiveness and tolerability for long-term use( Reference Freeman 1 , Reference Khalid and McMains 20 ).
Wheat allergy
WA is an IgE-mediated allergic reaction to the proteins found in wheat and other related cereals such as barley and rye( Reference Thomas and Bishop 14 ). A spectrum of different proteins of wheat has been involved in the presumed reaction, including gliadins, glutenins, serpins, thioredoxin, agglutinin and ATI( Reference Aziz, Dwivedi and Sanders 13 ). The reaction depends upon the route of exposure, but ingestion leads to the typical signs of food allergy, including skin, gastrointestinal and respiratory manifestations. WA can also be seen in the form of occupational asthma (baker’s asthma), rhinitis, wheat-dependent exercise-induced anaphylaxis and contact urticaria( Reference Elli, Branchi and Tomba 8 ).
The global prevalence of WA, including all forms, is approximately 4 %( Reference Sapone, Bai and Ciacci 21 ), and is more prevalent in children (2–9 %) than in adults (1–2 %)( Reference Sapone, Bai and Ciacci 21 ), where immediate WA is normally out-grown by the time the child reaches school age( Reference Elli, Branchi and Tomba 8 ). In children, WA is normally seen in combination with mild-to-moderate atopic dermatitis. In adults, the most common form of WA is wheat-dependent exercise-induced anaphylaxis, where the symptoms occur only in combination with physical activity after intake of wheat. The gastrointestinal symptoms are often mild and diffuse, normally seen as diarrhoea and/or bloating( Reference Elli, Branchi and Tomba 8 ).
WA is diagnosed with blood samples (increased serum-specific IgE levels and components against wheat), positive skin prick test with wheat and/or provocation with wheat ingestion by oral open and/or placebo-controlled food challenges. The only treatment for WA is, as with CD, a strict GFD to avoid the proteins found in wheat, barley and rye( Reference Aziz, Dwivedi and Sanders 13 ).
Non-coeliac gluten sensitivity
NCGS is defined as ‘a syndrome characterized by intestinal and extra-intestinal symptoms related to ingestion of gluten-containing food, in subjects that are not affected by either coeliac disease or wheat allergy’( Reference Catassi, Elli and Bonaz 9 , Reference Catassi, Bai and Bonaz 10 ). The terminology is somewhat debated, due to the fact that gluten is probably not the only protein involved in the development of symptoms( Reference Catassi, Elli and Bonaz 9 ). There is an ongoing discussion of the use of different terminologies as to what may entail a more accurate description, including terms such as wheat intolerance syndrome, non-coeliac wheat sensitivity or non-coeliac wheat protein sensitivity( Reference Catassi, Bai and Bonaz 10 , Reference Jericho, Assiri and Guandalini 22 , Reference De Giorgio, Volta and Gibson 23 ).
It has been suggested that NCGS may not exist and rather may have formed from the gluten hysteria seen in the media during the last several years, where a GFD has been promoted as a super-healthy diet solving almost ‘all’ health problems( Reference Elli, Roncoroni and Bardella 24 ). This has resulted in gluten being given an undeserved bad reputation, and made a large proportion of the population switch to a GFD without necessarily being allergic or intolerant. Many of these individuals without a diagnosis claim that they feel more fit and energetic when avoiding gluten and a GFD has been proposed as a solution to all intestinal as well as extra-intestinal afflictions. As a result of this, the accessibility and consumption of gluten-free products has become more prevalent( Reference De Giorgio, Volta and Gibson 23 ). Estimates show that between 10 and 20 % of all individuals in the USA and Australia are now avoiding gluten in their diet( Reference Makharia, Catassi and Makharia 6 ).
It is still uncertain whether NCGS truly is a clinical entity separated from irritable bowel syndrome (IBS) (Fig. 1). On one hand, some experts suggest that NCGS is a subgroup of IBS because of their overlapping symptoms, and that fermentable oligo-, di-, monosaccharides and polyols (FODMAPs) may play a key role in the trigger of symptoms, rather than gluten alone( Reference Vazquez-Roque and Oxentenko 25 ). On the other hand, recent studies have indeed provided evidence supporting the assumption that a specific, gluten- or wheat-induced reaction in some patients exists( Reference De Giorgio, Volta and Gibson 23 , Reference Vazquez-Roque and Oxentenko 25 , Reference Capannolo, Viscido and Barkad 26 ).
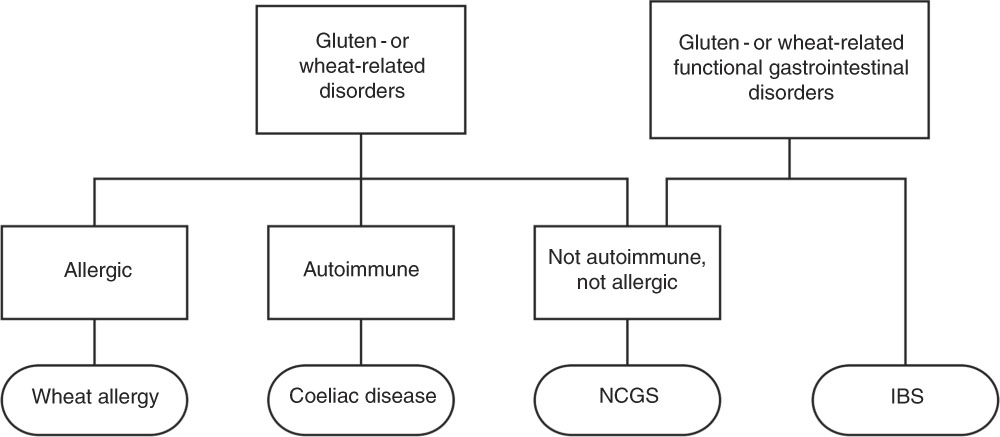
Fig. 1 Diet-evoked functional gastrointestinal disorders. NCGS, non-coeliac gluten sensitivity; IBS, irritable bowel syndrome.
Pathophysiology
The pathology and mechanisms behind NCGS are largely unknown, besides the known differences from CD and WA, regarding lack of typical immunological and allergic responses and lack of obvious damage to the gut. The literature suggests that there is a multifactorial process involved in the development of the condition, whereby gluten, FODMAPs (in particular fructans found in wheat and rye) and ATI have all been implicated as possible triggers( Reference Elli, Roncoroni and Bardella 24 , Reference Elli, Tomba and Branchi 27 – Reference Fasano, Sapone and Zevallos 29 ).
Alteration in the gut mucosal gene expression, seen as high expression of claudin-4 compared with controls, has been found in patients with NCGS. The up-regulation of claudin-4 was associated with reduction of T-regulatory cell marker FoxP3 relative to controls, as well as an increased expression of toll-like receptor-2. These mechanisms suggest that the intestinal innate immune system has a role in the development of NCGS, without any involvement of the adaptive immune response, making it different from the immunological response seen in CD( Reference Catassi, Bai and Bonaz 10 , Reference Vazquez-Roque and Oxentenko 25 , Reference Sapone, Lammers and Mazzarella 30 ). A study investigating the intestinal cell damage and systemic immune activation in individuals with suspected NCGS made observations that suggested that translocation of microbial products from the gastrointestinal tract may contribute to the innate and adaptive immune activation seen in wheat-sensitive subjects( Reference Uhde, Ajamian and Caio 31 ).
It is assumed that the events in the gut mucosa leading to NCGS are not the same as in the gut of a CD patient in response to gluten peptides. In patients with NCGS, there are no signs of inflammation when triggered with gliadin, and no activation of basophils( Reference Bucci, Zingone and Russo 32 ). In a study comparing biopsy materials from patients with CD and NCGS exposed to four slices of gluten-containing bread for 3 d, the production of cytokines was clearly increased in the CD group, whereas in the NCGS group only a slight increase of the cytokine interferon-γ was reported( Reference Husby and Murray 33 , Reference Brottveit, Beitnes and Tollefsen 34 ).
In vivo and in vitro studies suggest that wheat ATI could be a trigger for the innate immune response in the intestinal monocytes, macrophages and dendritic cells that eventually could lead to NCGS( Reference Catassi, Bai and Bonaz 10 , Reference Fasano, Sapone and Zevallos 29 , Reference Schuppan, Pickert and Ashfaq-Khan 35 ). The immune response is linked to the activation of the toll-like receptor-4 complex in the above-mentioned cells, leading to the release of pro-inflammatory chemokines and cytokines, causing intestinal inflammation( Reference Schuppan, Pickert and Ashfaq-Khan 35 , Reference Junker, Zeissig and Kim 36 ). ATI are a group of five (or more) homologous low-molecular-weight proteins, working as naturally occurring pesticides in wheat( Reference Vazquez-Roque and Oxentenko 25 ). They are highly resistant to intestinal proteolysis, which facilitates the maintenance of the ability to activate toll-like receptor-4 throughout oral ingestion and intestinal passage( Reference Fasano, Sapone and Zevallos 29 ).
ATI are known to be the main contributor allergen in baker’s asthma and it has been suggested that ATI can worsen the symptoms in subjects with pre-existing inflammatory disease, such as patients suffering from autoimmune diseases and/or chronic inflammation( Reference Catassi, Bai and Bonaz 10 , Reference Schuppan, Pickert and Ashfaq-Khan 35 ). Whereas gluten constitutes 80–90 % of the total protein in wheat, ATI only make up 2–4 %. An average consumption of wheat flour (150–250 g/d) constitutes an exposure of 0·5–1 g ATI per d. The co-existence of ATI in the grain endosperm with the gluten protein network results in a diet free from gluten always also being free from ATI( Reference Makharia, Catassi and Makharia 6 ). Further evidence, especially randomised double-blind placebo-controlled (DBPC) trials in suspected NCGS patients, is required to confirm this potential role of ATI.
Prevalence
NCGS has been reported in individuals with and without the HLA-DQ2/8 genotype. Currently, the data report that about 50 % of those suffering from NCGS have the HLA-DQ2/8 genotypes, a prevalence that is slightly higher than the normal population( Reference Ludvigsson, Leffler and Bai 4 , Reference Catassi, Bai and Bonaz 10 , Reference Aziz, Dwivedi and Sanders 13 ). The overall prevalence of NCGS in the general population is unknown, because, first, many patients are self-diagnosed and begin following a GFD without clinical support and guidance (to ensure definitive exclusion of CD), and, second, due to the lack of standardised international diagnostic criteria or biomarkers( Reference Catassi, Bai and Bonaz 10 , Reference Vazquez-Roque and Oxentenko 25 , Reference Volta, Pinto-Sanchez and Boschetti 37 ). It has been suggested that individuals suffering from NCGS constitute a larger group than those diagnosed with CD or WA, and that it globally affects a large proportion of the population ranging greatly from 0·6 to 13 %( Reference Aziz, Dwivedi and Sanders 13 ). The disorder seems to be more prevalent in females and in young to middle-aged adults( Reference Fasano, Sapone and Zevallos 29 , Reference Volta, Bardella and Calabrò 38 ). However, further research characterising this group will allow better diagnostic criteria and therefore more accurate prevalence data.
Symptoms
Common reported symptoms of NCGS are the same of those seen in patients with IBS( Reference Catassi, Elli and Bonaz 9 , Reference Catassi, Bai and Bonaz 10 ). Current literature suggests that the majority of patients suffering from IBS are intolerant to one or more food items, and show improvement of symptoms when reducing the FODMAP content in their diet( Reference Marsh, Eslick and Eslick 39 – Reference Hustoft, Hausken and Ystad 41 ). Patients with NCGS often report common IBS-like gastrointestinal symptoms such as abdominal pain and bloating, altered bowel habits (diarrhoea, constipation or mixed form), nausea and reflux, as well as extra-intestinal symptoms including headache, fibromyalgia, fatigue, anxiety, foggy mind, joint pain, disturbed sleep pattern, weight gain or weight increase, depression, skin rash and dermatitis( Reference Catassi, Elli and Bonaz 9 , Reference Catassi, Bai and Bonaz 10 , Reference Khalid and McMains 20 , Reference De Giorgio, Volta and Gibson 23 , Reference Peters, Biesiekierski and Yelland 42 ). The extra-intestinal symptoms are not explained by the known mechanisms of FODMAPs, suggesting that these sensations may be a potential specific reaction to gluten( Reference Peters, Biesiekierski and Yelland 42 ). Cutaneous manifestations and skin lesions are reported in a great proportion of patients with NCGS, and are reported to improve when on a GFD( Reference Bonciolini, Bianchi and Del Bianco 43 , Reference Picarelli, Borghini and Di Tola 44 ). The symptoms normally occur a short time after ingestion of gluten, lasting from hours to a few days( Reference Catassi, Elli and Bonaz 9 ).
Several studies suggest that there is a relationship between NCGS and neuropsychiatric disorders, highlighting autism spectrum disorders and schizophrenia( Reference Catassi, Elli and Bonaz 9 , Reference Catassi, Bai and Bonaz 10 , Reference Czaja-Bulsa 45 ). This hypothesis is linked to the assumption of the phenomenon ‘leaky-gut syndrome’, involving the brain–gut axis. Assuming an existence of an increased intestinal permeability (referred to as ‘leaky-gut syndrome’), gluten and gluten-like peptides are allowed to enter the bloodstream and cross the blood–brain barrier and affect the endogenous opioid system and neurotransmission in the nervous system( Reference Catassi, Elli and Bonaz 9 , Reference Czaja-Bulsa 45 , Reference de Magistris, Familiari and Pascotto 46 ). More specifically, by interacting with opioid brain receptors, gluten peptides, referred to as gluten ‘exorphins’, are thought to affect an individual’s behaviour, or trigger activation of immune cells migrating to the brain and causing neuro-inflammation. It is also suggested that gluten can be related to depression and other mental health effects by altering serotonin production. As most serotonin is derived from the gastrointestinal tract, it may be possible that gluten-containing foods influence serotonin production; however, the mechanisms remain unclear( Reference Biesiekierski and Iven 47 ).
Diagnosis and treatment
The current diagnosis of NCGS is often based on the patient’s own suspicion, where they believe their symptoms are directly linked to gluten ingestion and report rapid improvement of symptoms after excluding gluten from their diet( Reference Elli, Roncoroni and Bardella 24 ). There are no reproducible specific biomarkers or findings in a duodenal biopsy that have been identified to establish the diagnosis of NCGS. To confirm the diagnosis, CD and WA must be excluded. It is important to exclude CD while the patient is still on a gluten-containing diet( Reference Catassi, Bai and Bonaz 10 ). Table 1 shows an overview and comparison of the key factors defining the range of gluten-related disorders( Reference Tovoli, Masi and Guidetti 2 , Reference Ludvigsson, Leffler and Bai 4 , Reference Catassi, Elli and Bonaz 9 ).
Table 1 Comparison of the gluten-related disorders

CD, coeliac disease; WA, wheat allergy; NCGS, non-coeliac gluten sensitivity; EMA, endomysial antibodies; tTG, tissue transglutaminase; DGP, deaminated gliadin peptides; AGA, anti-gliadin antibodies; HLA, human leucocyte antigen; GFD, gluten-free diet.
A newly developed standardisation of a diagnostic gluten challenge submitted by ‘the Salerno Experts’ criteria’ has suggested a set of guidelines on how to manage the diagnosis in these patients( Reference Catassi, Elli and Bonaz 9 ). To confirm the presence of NCGS, the patient must follow a DBPC oral gluten challenge and be able to identify when receiving gluten( Reference Catassi, Elli and Bonaz 9 , Reference Catassi, Bai and Bonaz 10 , Reference Elli, Tomba and Branchi 27 ). A DBPC food challenge is regarded as the ‘gold standard’ for investigating food allergies and intolerances. A DBPC food challenge is performed after a phase of an elimination diet where the patient avoids the food item(s) suspected to give a reaction, in this case gluten( Reference Arslan, Kahrs and Lind 48 ). Several studies have implemented a DBPC gluten challenge to investigate the effect of gluten in patients with suspected NCGS, but have shown a lack of a gluten-specific response, as well as a high nocebo response to the placebo provocations( Reference Molina-Infante, Santolaria and Sanders 49 ).
The presence of the antibody IgG anti-gliadin antibody in patients with NCGS was recently suggested as a possible biomarker, found in serum in about 50 % of the patients diagnosed with NCGS( Reference Catassi, Elli and Bonaz 9 ). However, this antibody is not specific for NCGS, as it is also found in patients with CD, IBS, autoimmune liver disorder and connective tissue disease, as well as some healthy controls( Reference Volta, Bardella and Calabrò 38 ). The lack of diagnostic accuracy and the weak correlation emphasise the need for more research( Reference Mocan and Dumitrascu 11 , Reference Infantino, Manfredi and Meacci 50 ).
A newly published study evaluating intestinal, systemic and oral gluten-related alterations in patients with NCGS presented a possible new diagnostic test of an oral mucosa patch test with gluten. However, the study sample was small and further investigations are needed to evaluate the reproducibility and clinical relevance of this method( Reference Picarelli, Borghini and Di Tola 44 ).
There are no specific guidelines available for the treatment of NCGS. Although a GFD is practised, the threshold for gluten tolerance in these patients remains unknown, and it has been suggested that individual levels of gluten tolerance may vary in the subjects affected( Reference Volta, Pinto-Sanchez and Boschetti 37 ). Because the pathology of disease is unknown at this point of time, it is not known whether a lifelong GFD is necessary, whether the condition is fluctuating or whether food components other than gluten may also need to be avoided due to an accumulative effect( Reference Foschia, Horstmann and Arendt 5 , Reference Catassi, Elli and Bonaz 9 , Reference Aziz, Dwivedi and Sanders 13 , Reference Khalid and McMains 20 , Reference Vazquez-Roque and Oxentenko 25 ). A better understanding of food intolerance and hypersensitivity related to wheat and gluten, as well as specific biomarkers to diagnose NCGS are needed for the development of more definitive dietary guidelines for patients with NCGS( Reference Volta, Pinto-Sanchez and Boschetti 37 ).
Nocebo effects
In a DBPC food challenge, participants often experience a negative placebo effect, given that they know they may receive a test material they believe as problematic. This is called the nocebo effect, meaning that the participants are expecting to experience unpleasant symptoms, because they already have a biased expectation about the consequence of eating gluten. It is likely to think that most patients suffering from suspected NCGS are to some degree influenced by a nocebo effect( Reference Picarelli, Borghini and Di Tola 44 ). As already mentioned, gluten has received an undeserved bad reputation during the last decade, and the GFD has been promoted as a diet solving both extra-intestinal and intestinal afflictions( Reference Elli, Roncoroni and Bardella 24 ). It is therefore likely to assume that a great proportion of patients reporting symptoms related to gluten in fact experience a nocebo effect rather than symptoms physiologically caused by the consumption of gluten, and that the nocebo effect can explain partly the high placebo response seen in many DBPC gluten challenges( Reference Picarelli, Borghini and Di Tola 44 ). Also, it can arguably be problematic to avoid bias when implementing a ‘similar’ gluten-containing and gluten-free test substance in a double-blind food trial, due to the known effect of gluten in foods. It has to be considered that both appearance, colour, texture and elasticity of the food can be affected in the absence of gluten; thus the failure to mask treatment is a potential bias. This is an important limitation for all studies into NCGS using real foods as test substances.
Recent evidence from clinical studies on non-coeliac gluten sensitivity
Several interventional studies have recently investigated the effect of a gluten challenge in patients suspected to have NCGS, to see whether gluten can specifically induce symptoms( Reference Peters, Biesiekierski and Yelland 42 , Reference Picarelli, Borghini and Di Tola 44 , Reference Di Sabatino, Volta and Salvatore 51 – Reference Skodje, Sarna and Minelle 55 ). The current studies presented are summarised in Table 2.
Table 2 Comparison of current primary research on non-coeliac gluten sensitivity, summarising sample size, study design, test material, amount of gluten, placebo material, duration, days of wash out and primary end-point

DBPCT, double-blind, placebo-controlled trial; GF, gluten-free; STPI, State Trait Personality Inventory.
A study from Australia, conducted by Biesiekierski et al. (2011), was the first study resulting in evidence towards the existence of NCGS( Reference Biesiekierski, Newnham and Irving 52 ). This DBPC trial of a single dose of gluten (16 g) randomised thirty-four patients on a GFD to receive two slices of bread and one muffin per d, either with (16 g in total per d) or without (0 g) gluten, for 6 weeks. The gluten group showed more severe gastrointestinal symptoms and increased tiredness compared with the placebo group within the first week, but no differences were seen for intestinal permeability, C-reactive protein or faecal lactoferrin( Reference Biesiekierski, Newnham and Irving 52 ). Although these results were considered the first evidence for the existence of NCGS, the results from a controlled dietary study published by the same group a couple of years later did not support these findings.
The follow-up study (2013) implemented a 2-week run-in period on a low-FODMAP diet. Patients (n 37) with NCGS and IBS symptomatically controlled on a GFD underwent a DBPC randomised cross-over trial of placebo, low gluten (2 g/d) or high gluten (16 g/d) for 1 week, followed by a 2-week wash-out period before crossing over to the next diet. Symptoms significantly improved as a consequence of reducing the FODMAP content in the diet, but significantly worsened to a similar degree during each dietary treatment, irrespective of content in the diet (placebo/low-gluten/high-gluten). Only six participants (16 %) had symptoms significantly elevated on the high-gluten diet( Reference Biesiekierski, Peters and Newnham 53 ). This study was important for highlighting that other dietary triggers play a role in NCGS, given that when patients were instructed to follow a diet low in FODMAPs on top of their usual GFD during the run-in period, there was significant improvement in symptoms for all participants after 2 weeks( Reference Biesiekierski, Peters and Newnham 53 ).
Peters et al. ( Reference Peters, Biesiekierski and Yelland 42 ) conducted a study to investigate the effect of gluten on mental state in patients with suspected NCGS. The twenty-two participants randomly received one of three dietary interventions for 3 d (16 g gluten, 16 g whey protein, 16 g placebo) followed by at least 3 d of wash out before crossing over to the next diet. They found that short-term exposure to gluten induced feelings of depression, but no gluten-specific induction of gastrointestinal symptoms was identified.
Di Sabatino et al. ( Reference Di Sabatino, Volta and Salvatore 51 ) reported in 2015 a significant increase of symptoms related to gluten in a double-blind cross-over study of a group of sixty-one patients with suspected NCGS. The participants were given gluten (4·375 g/d) v. placebo (rice starch) in the form of gastro-soluble capsules for 1 week, followed by 1 week of wash out before crossing over to the next challenge period. The primary outcome, measured as the change in overall symptoms (both intestinal and extra-intestinal), showed that gluten significantly (P=0·034) increased the overall symptoms compared with placebo.
A randomised clinical cross-over study in thirty-five subjects on a GFD, conducted by Zanini et al. ( Reference Zanini, Basche and Ferraresi 54 ), reported that a gluten challenge induced recurrence of symptoms in only one-third of the patients meeting the diagnostic criteria for NCGS. The participants were randomised to receive either gluten-containing or gluten-free flour for 10 d, followed by a 2-week wash-out period before crossing over to the next 10 d. The main outcome was the participants’ ability to identify which flour contained gluten. Correct identification of the gluten was achieved by only twelve participants (34 %), who thus were classified as having NCGS( Reference Zanini, Basche and Ferraresi 54 ).
Picarelli et al. ( Reference Picarelli, Borghini and Di Tola 44 ) reported in 2016 that in a group of sixty patients with suspected NCGS, forty patients showed positive results when exposed to an oral mucosa patch test for gluten, indicating such a test as a possible specific tool for NCGS diagnosis. Interestingly, no significant difference between severity of symptoms during a 1 d oral gluten/placebo challenge (using similar croissants, 10 g gluten (n 13)/0 g gluten (n 13) were seen in twenty-six of these patients.
Elli et al. ( Reference Elli, Tomba and Branchi 27 ) were the first to conduct a study according to the previously mentioned Salerno Experts’ criteria for NCGS. They identified a group of patients with NCGS by conducting a DBPC gluten challenge in ninety-eight patients with functional gastrointestinal symptoms. Only 14 % of the patients showed a symptomatic relapse during the gluten challenge, and were therefore identified as patients with NCGS( Reference Elli, Tomba and Branchi 27 ).
Skodje et al. ( Reference Skodje, Sarna and Minelle 55 ) reported recently that fructans, rather than gluten, induce symptoms in patients with self-reported NCGS. Participants were randomly assigned to receive gluten-containing bars (5·7 g gluten), fructan-containing bars (2·1 g fructans) or placebo bars for 7 d, before crossing over to the next diet, after at least 7 d wash out. They found no differences in symptoms between gluten and placebo, but a significant increase in symptoms after the challenge with fructans( Reference Skodje, Sarna and Minelle 55 ).
Recently, we have also performed a randomised, oral DBPC gluten challenge in a group of twenty patients on a GFD and suspected to have NCGS( Reference Dale, Hatlebakk and Hovdenak 56 ). The participants went through four periods of double-blinded challenge, two with gluten and two with placebo in a randomised order. They were instructed to eat two muffins per d (11 g gluten or none for the placebo arm) for 4 d, followed by 3 d of wash out, before crossing over to the next period. The results showed no specific effect of gluten when evaluating symptom scores during gluten and placebo challenges. Four out of twenty patients were diagnosed with NCGS due to their correct identification of the gluten-containing muffins, but interestingly, these four patients did not show any specific effect to gluten when evaluating their symptom score. Given that all of the study participants showed similar high symptom scores during both gluten and placebo challenges, it is likely that symptoms experienced by this group of patients were triggered by something other than gluten and may have been influenced by the nocebo effect( Reference Dale, Hatlebakk and Hovdenak 56 ).
A newly published review by Lionetti et al. (2017) presented a meta-analysis of different studies evaluating the effect of a re-challenge with gluten in patients with suspected NCGS( Reference Lionetti, Pulvirenti and Vallorani 57 ). After comparing eleven different studies, they found that the prevalence of NCGS is low following re-challenge with gluten, and that the percentage of relapse after a gluten or a placebo challenge is similar. However, they emphasise that a higher number of patients will be correctly diagnosed with NCGS when applying the Salerno criteria( Reference Lionetti, Pulvirenti and Vallorani 57 ). These findings are supported by another recent published meta-analysis by Molina-Infante et al. ( Reference Molina-Infante and Carroccio 58 ) concluding that suspected NCGS is confirmed in few patients after gluten challenge in DBPC trials. This meta-analysis highlighted that only 16 % of NCGS patients show gluten-specific symptoms, and that as many as 40 % of these patients have similar or increased symptoms to placebo, impairing the supposition of gluten as the main trigger of symptoms( Reference Molina-Infante and Carroccio 58 ).
Discussion of current research
Current studies evaluating the effect of NCGS show highly variable results and no clear indication regarding the existence of NCGS as a unique diagnosis. The lack of difference in symptoms between placebo and gluten challenges in most studies is a significant problem, giving strength to the assumption that gluten may not be the main trigger of symptoms in most patients. The high nocebo response indicates that other triggers are involved, and that a DBPC trial may not be the best way of diagnosing patients with NCGS with a known high nocebo response. As highlighted in the most recently published research, it is highly relevant to further evaluate the effect of fructans and a (customised) low-FODMAP diet in this group of patients. Recent evidence points towards non-coeliac wheat sensitivity as a more proper term.
Implications of following a gluten-free diet
The market of gluten-free products is constantly growing as a consequence of the increased proportion of the population following a GFD. A strict GFD is the only safe and recommended treatment for CD and WA, whereas for patients suffering from NCGS and IBS the advice is not as well defined( Reference Tovoli, Masi and Guidetti 2 ).
Bread is a staple food consumed daily all over the world, and constitutes the major source of gluten in a normal subject’s everyday diet. The dough characteristics and bread quality can mainly be attributed to the presence of gluten, and despite the improved quality of gluten-free breads during the last few years, most products continue to be described as low quality, with a lower nutritional value in gluten-free products when compared with their normal, gluten-containing products( Reference Foschia, Horstmann and Arendt 5 ).
Furthermore, when removing gluten from the diet, many individuals consume less nutritional diversity than before, and it is important to be aware of consequent deficiencies. Gluten-free products are often low in fibre, Fe, Zn, Mg and vitamin B. Therefore, common gluten-free alternative products are often fortified, to reduce the risk of deficiencies. Starch-rich foods should be used as alternatives to gluten-containing foods include rice, maize, potato, quinoa, amaranth, buckwheat, millet, nuts and legumes. The transition to a strict GFD should involve nutritional education and guidance from a clinical dietitian to ensure that the diet is adequate and fulfils all nutritional needs( Reference Kupper 59 ).
Irritable bowel syndrome and non-coeliac gluten sensitivity: a complex relationship
It is likely that the symptoms seen in patients with suspected NCGS cannot be attributed to gluten alone. There is compelling evidence that other substances in wheat are involved, especially the role of the carbohydrates found in wheat( Reference Biesiekierski, Muir and Gibson 7 , Reference El-Salhy, Hatlebakk and Gilja 60 ). Fructans (a group of oligosaccharides) is the term of a non-digestible carbohydrate found in wheat, barley and rye, and is regarded as a part of the collective term ‘FODMAP’ (fermentable oligo-, di-, monosaccharides and polyols)( Reference El-Salhy, Hatlebakk and Gilja 60 , Reference Biesiekierski, Rosella and Rose 61 ).
FODMAPs are poorly absorbed in the small intestine, and can cause increased gas production and luminal distention, as well as intestinal osmolality due to fermentation by the colonic bacteria( Reference Halmos, Power and Shepherd 62 ). The implementation of a low-FODMAP diet usually involves avoiding or limiting the intake of high-FODMAP foods in the diet, with the intention of limiting the delivery of osmotically active and fermentable substrates to the intestine, thereby minimising luminal distention and gastrointestinal symptoms( Reference Hustoft, Hausken and Ystad 41 ). A diet low in FODMAPs has been shown to reduce symptoms in up to 80 % of patients with IBS( Reference Marsh, Eslick and Eslick 39 , Reference Hustoft, Hausken and Ystad 41 , Reference Halmos, Power and Shepherd 62 ).
A diet free from gluten-containing food will automatically lead to a lower intake of FODMAPs due to the avoidance of fructans found in gluten-containing foods (wheat, rye, barley). Therefore, the potential gastrointestinal symptom relief experienced by patients with suspected NCGS following a GFD can potentially be due to the reduction of FODMAPs and not gluten in particular, making it difficult to distinguish between the diagnosis of IBS and NCGS( Reference Biesiekierski, Peters and Newnham 53 , Reference Rajilic-Stojanovic, Jonkers and Salonen 63 ). However, most patients suffering from IBS will experience a reduction in symptoms by excluding wheat (fructans) from the diet, but also by avoiding other high FODMAP-containing food, whereas patients with suspected NCGS report reduction in symptoms when avoiding only gluten (wheat, rye, barley)( Reference De Giorgio, Volta and Gibson 23 , Reference El-Salhy, Hatlebakk and Gilja 60 ).
When evaluating the difference between a food intolerance (seen as gastrointestinal symptoms in IBS patients eating FODMAPs) and food hypersensitivity (potentially seen as an immune response to antigens derived from nutrients that triggers both gastrointestinal and extra-gastrointestinal symptoms in NCGS), it is likely that IBS and NCGS are different conditions, with overlapping and similar characteristics (Fig. 2)( Reference Makharia, Catassi and Makharia 6 , Reference Husby and Murray 33 ).
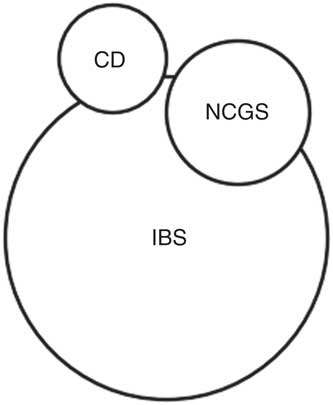
Fig. 2 Possible relationship and overlap between coeliac disease (CD), irritable bowel syndrome (IBS) and non-coeliac gluten sensitivity (NCGS).
Future research directions
There are several concerns for well-designed dietary trials in patient groups with suspected NCGS. As already mentioned and highlighted in previous research, it is a highly suggestible group who often experience a high nocebo response. The selection of patients to include is a challenge due to the lack of standard guidelines defining NCGS, including adequate exclusion of CD, the evaluation of the implemented GFD as well as the great variance in symptom definitions. The method of gluten challenge needs to be standardised due to the high variation of the provocation substance (food v. capsule) and amount of gluten in the test substance implemented in studies this far, making the comparison of results difficult. The control of confounding dietary factors is important in this group of patients, due to the well-known effect of FODMAPs( Reference Biesiekierski, Peters and Newnham 53 ). Also, a standardised and validated cut-off limit for a significant reduction or increase in symptoms, indicating positive/negative test result and what may be clinically relevant according to symptom questionnaires, needs to be developed( Reference Catassi, Elli and Bonaz 9 ).
For further clinical studies on NCGS, it is necessary not only to focus on the effect of gluten in this group of patients, but also to investigate the effect of wheat as a whole, including all components that might contribute to intestinal and extra-intestinal symptoms. One suggestion would be to separate between provocations using gluten, fructans and ATI in the same group of patients when using a DBPC design, and further investigate the accumulative effect of FODMAPs and gluten.
Conclusions
While CD and WA are diagnoses with well-known pathophysiology and mechanisms, established clinical procedures and life-long treatment with a GFD, NCGS is still a new entity with unknown mechanisms and a need for validation. For the development of better diagnostic criteria and optimisation of clinical care, it has to be considered that gluten is most likely not the only substance in wheat that triggers symptoms. Other wheat components such as fructans (FODMAPs) and ATI need to be better investigated as potential pathogenic factors. More clinical research is needed to answer the question of whether NCGS really does exist. Based on current evidence the existence of NCGS as a clinical entity remains unestablished, and non-coeliac wheat sensitivity can probably be used as a more proper term.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
All authors contributed to the paper. H. F. D wrote, and J. B. and G. A. L. corrected the manuscript.
There were no conflicts of interest.