Article contents
In situ characterizations of photoelectrochemical cells for solar fuels and chemicals
Published online by Cambridge University Press: 27 October 2020
Abstract
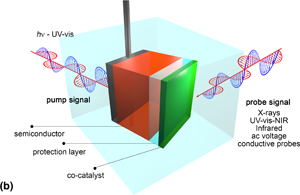
Environmental concerns deriving from fossil fuel dependency are driving an energy transition based on sustainable processes to make fuels and chemicals. Solar hydrogen is the pillar of this new green economy, but the technological readiness level of PV electrolysis and direct photoelectrochemical (PEC) electrolysis are still too low to allow broad commercialization. Direct conversion through PEC technology has more potential in the medium–long term but must be first guided by the scientific enhancements to improve device efficiencies. For this purpose, in situ and operando photoelectrochemistry will guide the discovery of new materials and processes to make solar fuels and chemicals in PEC cells.
The use of advanced in situ and operando characterizations under working photoelectrochemical (PEC) conditions is reviewed here and anticipated to be a fundamental tool for achieving a basic understanding of new PEC processes and for enabling the large-scale development of PEC technology by 2050, thus delivering fuels and chemicals having zero (or negative) carbon footprint. Hydrogen from solar water splitting is the most popular solar fuel and can be mainly produced by indirect photovoltaic-driven electrolysis (PV electrolysis) and direct photoelectrochemistry. Although PV electrolysis has already been developed on a level of MW-scale pilot plants, PEC technology, which is much less mature, holds several advantages in the long term over PV-electrolysis systems. The key enabling feature to developing PEC technology is the improvement of the photoelectrode materials which are responsible for the absorption of light, and transport of the photo-generated charge carriers to drive the electrochemical surface reaction. These processes are often complex and multistep, spanning multiple timescales and following the simultaneous detection of photoelectrodes modification and formation of reaction intermediates/products can be achieved using eight well-known characterization techniques here presented.
- Type
- Review Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 12
- Cited by



