Article contents
Three-dimensionally ordered macroporous TiO2 electrodes: Fabrication of inverse TiO2 opals for pore-size-dependent characterization
Published online by Cambridge University Press: 28 September 2012
Abstract
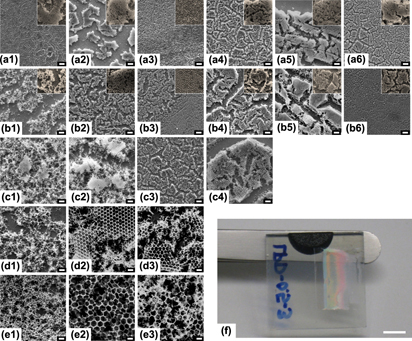
The increased use of viscous electrolytes in dye-sensitized solar cells (DSSCs) has revitalized the interest in three-dimensionally ordered macroporous (3DOM) structures as potential electrodes. The simplest approach to a 3DOM structure, such as inverse opals, is colloidal assembly followed by precursor infiltration and calcination. However, current assembly methods are often optimized for very narrow particle size ranges due to the colloidal forces specific to the method used. Using five particle sizes, ranging from 0.5 to 10 μm, we have studied the particle-size dependence of six commonly used colloidal assembly methods and its effect on the resulting inverse opal structure using scanning electron microscopy and Fast Fourier Transforms. Our results indicate a clear correlation between particle size and colloidal assembly method. The information provided by our study will enable systematic studies on inverse opal TiO2 electrodes with various pore sizes, in which the influence of the inverse opal preparation methods used is minimized.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 369 - 377
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 6
- Cited by




