Article contents
Thermodynamic modeling and characterizations of Al nanoparticles produced by electrical wire explosion process
Published online by Cambridge University Press: 20 January 2017
Abstract
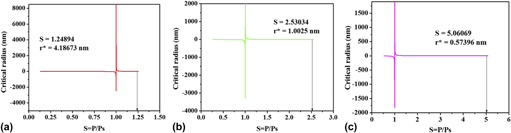
Aluminum (Al) nanoparticles are synthesized by wire explosion process (WEP) in an inert ambience of argon. Thermodynamic analysis and structural characterization of nano Al particles are made in the present work. Transmission electron microscopy (TEM) characterization has shown that the Al nanoparticles produced are spherical in shape and it follows a lognormal distribution. A unimodal size dependent thermodynamic model is formulated to understand the size dependent thermal behavior of aluminum nanoparticles. Three different melting modes such as, homogeneous melting mode (HMM), liquid skin melting (LSM) and liquid nucleation and growth (LNG) are assumed to understand the melting behavior of aluminum nanoparticles synthesized by the WEP process. The effect of saturation ratio on the nucleation rate and the impingement factor is also discussed. The size dependent melting and enthalpy of fusion of Al nanoparticles predicted by thermodynamic model are in tandem with the DSC results.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
References
REFERENCES
A correction has been issued for this article:
- 10
- Cited by
Linked content
Please note a has been issued for this article.





