Article contents
Synthesis of novel shape-stabilized phase change materials with high latent heat and low supercooling degree for thermal energy storage
Published online by Cambridge University Press: 12 April 2019
Abstract
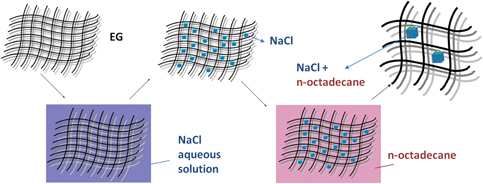
In this work, a novel shape-stabilized phase change material, composed of n-octadecane, expanded graphite (EG), and sodium chloride (NaCl), was prepared by a convenient method. In the composite, EG was used as the matrix material and NaCl served as the nucleating agent. Effects of the additional amount of NaCl on the thermal properties of the composite were investigated by DSC and TG. The melting and crystallization enthalpies of the composite are −160.23 J/g and 162.80 J/g, respectively; the supercooling degree of the composite decreased to 3.77 °C when compared to 7.58 °C of the pure n-octadecane. Furthermore, the thermal cycling performances became better, and the thermal decomposition temperature improved to 150 °C. The composite exhibited high latent heat, low supercooling degree, good thermal cycling performance, and enhanced thermal stability, making it a potential material for the thermal energy storage application in the field of thermal regulation.
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 19: Focus Issue: Thermodynamics of Complex Solids , 14 October 2019 , pp. 3263 - 3270
- Copyright
- Copyright © Materials Research Society 2019
References
- 7
- Cited by




