Article contents
Study of the impregnation of NiMo assisted by chelating agents for hydrodesulfurization-supported catalysts over mesoporous silica
Published online by Cambridge University Press: 21 September 2018
Abstract
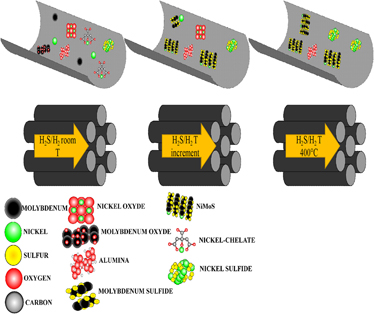
Molybdenum sulfide hydrotreating catalysts promoted with nickel over tridimensional mesoporous silica (KIT-6 post synthesis modified with alumina) were prepared with three different chelating agents. Citric acid and EDTA (ethylenediaminetetraacetic acid) were used as typical chelates and the new suggestion, polyacrylic acid as a polymeric agent. The catalysts were synthesized by the incipient wetness impregnation method, and two different activation methods were applied to determine the correlation between the chelating agent and activation conditions. The beneficial use of chelating agents was evaluated in their performance on HDS (hydrodesulfurization) of DBT (dibenzothiophene). To determine the properties of catalysts, nitrogen physisorption, X-ray diffraction, HRTEM (high-resolution transmission electron microscopy), and TGA (thermogravimetric analysis) were used. The beneficial effect of chelating Ni during impregnation to avoid NiSx formation and thus promoting NiMoS arrangement was clearly observed in the catalytic HDS performance, and the TGA analysis of Ni-chelate complexes also confirms this theory. The catalyst with the best performance in the HDS reaction of DBT was the synthesized with citric acid and a slow rate temperature sulfidation.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 33 , Issue 21: Focus Issue: Catalytic Engineered Materials for Commercial and Industrial Energy Applications , 14 November 2018 , pp. 3604 - 3613
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 1
- Cited by




