Article contents
Silaffin primary structure and its effects on the precipitation morphology of titanium dioxide
Published online by Cambridge University Press: 11 May 2016
Abstract
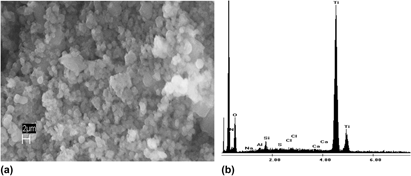
Inorganic oxides exhibit numerous applications influenced by particle size and morphology. While industrial methods for forming oxides involve harsh conditions, nature has the ability to form intricate structures of silicon dioxide (silica) using small peptides and polyamines under environmentally friendly conditions. Recent research has demonstrated that these biomaterials will precipitate other inorganic oxides, such as titanium dioxide (titania). Using the diatom-derived R5 peptide, new peptides with systematic changes (e.g., truncation and substitution) in the R5 primary structure were surveyed for reactivities and the impact on the morphology of the titania. The results demonstrated that (i) basic residues are vital to initiating the reaction, and a minimum local concentration is necessary to sustain the precipitation, (ii) residues containing hydroxyl side chains are important to imparting morphological control on the precipitate, and (iii) buffer conditions can dramatically alter both precipitation and morphology.
- Type
- Biomineralization and Biomimetics Article
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 4
- Cited by





