Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Cao, W.
and
Thomas, G.
1992.
HREM study of heteroepitaxial interfaces in the Ni.50Co.50O/Al2MgO4(100) system.
Proceedings, annual meeting, Electron Microscopy Society of America,
Vol. 50,
Issue. 1,
p.
374.
Ganapathi, S.K.
Spada, F.E.
and
Talke, F.E.
1992.
Residual stresses and their effect on wear behavior of polycrystalline zirconia overcoated disks.
IEEE Transactions on Magnetics,
Vol. 28,
Issue. 5,
p.
2533.
Thomas, G.
1993.
Electron microscopy of advanced magnets.
Ultramicroscopy,
Vol. 49,
Issue. 1-4,
p.
286.
Thomas, G.
1993.
Electron microscopy of nanostructured materials.
Nanostructured Materials,
Vol. 3,
Issue. 1-6,
p.
101.
Lederman, D.
Belanger, D. P.
Wang, J.
Han, S-J.
Paduani, C.
Ramos, C. A.
and
Nicklow, R. M.
1993.
Neutron and X-Ray Scattering Studies of (FeF2) M (CoF2)n Multilayers.
MRS Proceedings,
Vol. 313,
Issue. ,
Borchers, J. A.
Carey, M. J.
Erwin, R. W.
Majkrzak, C. F.
and
Berkowitz, A. E.
1993.
Spatially modulated antiferromagnetic order in CoO/NiO superlattices.
Physical Review Letters,
Vol. 70,
Issue. 12,
p.
1878.
Berry, S. D.
Lind, D. M.
Lochner, E.
Shaw, K. A.
Hilton, D.
Erwin, R. W.
and
Borchers, J. A.
1993.
Interfacial Exchange Coupling and the magnetization of Iron Oxide/Nickel Oxide Superlattices.
MRS Proceedings,
Vol. 313,
Issue. ,
Lind, D. M.
Tay, S.-P.
Berry, S. D.
Borchers, J. A.
and
Erwin, R. W.
1993.
Structural and magnetic ordering in iron oxide/nickel oxide multilayers by x-ray and neutron diffraction (invited).
Journal of Applied Physics,
Vol. 73,
Issue. 10,
p.
6886.
Fullerton, Eric E.
Cao, Wei
Thomas, Gareth
Schuller, Ivan K.
Carey, Matthew J.
and
Berkowitz, Ami E.
1993.
Quantitative characterization of epitaxial superlattices by x-ray diffraction and high resolution electron microscopy.
Applied Physics Letters,
Vol. 63,
Issue. 4,
p.
482.
Carey, M. J.
Berkowitz, A. E.
Borchers, J. A.
and
Erwin, R. W.
1993.
Strong interlayer coupling in CoO/NiO antiferromagnetic superlattices.
Physical Review B,
Vol. 47,
Issue. 15,
p.
9952.
Borchers, J. A.
Carey, M. J.
Berkowitz, A. E.
Erwin, R. W.
and
Majkrzak, C. F.
1993.
Propagation of antiferromagnetic order across paramagnetic layers in CoO/NiO superlattices.
Journal of Applied Physics,
Vol. 73,
Issue. 10,
p.
6898.
Lederman, D.
Ramos, C. A.
Jaccarino, V.
and
Cardy, J. L.
1993.
Finite-size scaling inFeF2/ZnF2superlattices.
Physical Review B,
Vol. 48,
Issue. 11,
p.
8365.
Fullerton, Eric E.
Conover, M. J.
Mattson, J. E.
Sowers, C. H.
and
Bader, S. D.
1993.
150% magnetoresistance in sputtered Fe/Cr(100) superlattices.
Applied Physics Letters,
Vol. 63,
Issue. 12,
p.
1699.
Cao, W.
Thomas, G.
Carey, M. J.
and
Berkowitz, A. E.
1994.
Microstructure of sputtered epitaxial Ni0.80Fe0.20/NixCO1−xO exchange coupled bilayers on α-Al2O3 (0001).
Journal of Electronic Materials,
Vol. 23,
Issue. 10,
p.
1043.
Thomas, Gareth
1994.
High resolution analyses and interfacial design of inorganic materials.
Scripta Metallurgica et Materialia,
Vol. 31,
Issue. 8,
p.
953.
1995.
Handbook of Inorganic Electrochromic Materials.
p.
527.
Egelhoff, W. F.
Ha, T.
Misra, R. D. K.
Kadmon, Y.
Nir, J.
Powell, C. J.
Stiles, M. D.
McMichael, R. D.
Lin, C.-L.
Sivertsen, J. M.
Judy, J. H.
Takano, K.
Berkowitz, A. E.
Anthony, T. C.
and
Brug, J. A.
1995.
Magnetoresistance values exceeding 21% in symmetric spin valves.
Journal of Applied Physics,
Vol. 78,
Issue. 1,
p.
273.
Egelhoff, W. F.
Chen, P. J.
Powell, C. J.
Stiles, M. D.
McMichael, R. D.
Lin, C.-L.
Sivertsen, J. M.
Judy, J. H.
Takano, K.
and
Berkowitz, A. E.
1996.
Growth of giant magnetoresistance spin valves using Pb and Au as surfactants.
Journal of Applied Physics,
Vol. 80,
Issue. 9,
p.
5183.
Thomas, Gareth
1996.
Electron microscopy and microanalysis of ceramics.
Journal of the European Ceramic Society,
Vol. 16,
Issue. 3,
p.
323.
Michel, R.P.
Chaiken, A.
Kim, Y.K.
and
Johnson, L.E.
1996.
NiO exchange bias layers grown by direct ion beam sputtering of a nickel oxide target.
IEEE Transactions on Magnetics,
Vol. 32,
Issue. 5,
p.
4651.
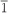 2) twin boundaries perpendicular to the oxide-substrate interface. The twin regions are approximately 30 nm in size and are uniformly distributed throughout the film. The epitaxial orientation of the monoxide films with respect to the substrate can be summarized by the relationships [111] monoxide // [0001] α−Al2O3, [1
2) twin boundaries perpendicular to the oxide-substrate interface. The twin regions are approximately 30 nm in size and are uniformly distributed throughout the film. The epitaxial orientation of the monoxide films with respect to the substrate can be summarized by the relationships [111] monoxide // [0001] α−Al2O3, [1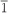 0] monoxide // [1
0] monoxide // [1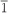 00] α−Al2O3, and [11
00] α−Al2O3, and [11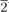 ] monoxide // [11
] monoxide // [11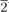 0] α−Al2O3.
0] α−Al2O3.



