Article contents
Preparation and photovoltaic properties of N-doped TiO2 nanocrystals in vacuum
Published online by Cambridge University Press: 08 January 2013
Abstract
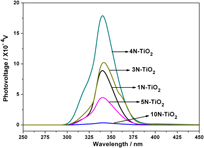
This work presents the preparation and characterization of N-doped TiO2 nanocrystals obtained by a solid-state reaction in vacuum with urea as the nitrogen source. The particle sizes of the products are smaller than 20 nm from the x-ray powder diffraction patterns and the transmission electron microscopy images. Different from the reported samples obtained in air or under dry N2 or NH3 gas flow, the doped nitrogen exists mainly as absorbed NOx groups but as smaller incorporated species in the nanocrystals, which is supported by the results from x-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, and ultraviolet–visible diffuse reflectance spectroscopy. Dependent on the nitrogen amount, the surface photovoltage (SPV) response reaches the maximum at the mediate molar ratio of 5:4 (urea to TiO2), which can be explained that proper nitrogen concentration can enhance the separation of the photogenerated carriers to improve the SPV intensity, but excess nitrogen can spread the impurity energy levels to narrow energy gaps, which reinforces the combination of the photogenerated electrons and holes and then decreases the SPV signal. The corresponding detailed discussion is also reported.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 468 - 474
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 2
- Cited by




