Article contents
Molten calcium–magnesium–aluminosilicate interactions with ytterbium disilicate environmental barrier coating
Published online by Cambridge University Press: 14 August 2020
Abstract
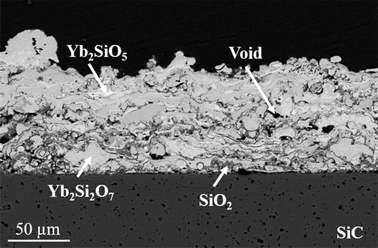
Thermochemical interactions between calcium–magnesium–aluminosilicate (CMAS) glass and an environmental barrier coating of ytterbium disilicate (Yb2Si2O7) and ytterbium monosilicate (Yb2SiO5) were investigated. Top coats were deposited by plasma spray-physical vapor deposition onto silicon carbide substrates. CMAS powder was prepared as a glass and cast into a tape to yield a CMAS loading of ~29 mg/cm2. Samples were heat treated with CMAS at 1300 °C for 1–10 h or at 1400 °C for 1 h in air. Polished specimen cross-sections were characterized using scanning electron microscopy, X-ray diffraction, X-ray energy-dispersive spectroscopy, and transmission electron microscopy to evaluate resulting microstructures, phases, and compositions at CMAS/Yb2Si2O7 interfaces. Coatings exposed at 1300 °C—10 h and 1400 °C—1 h were fully infiltrated and compromised by CMAS. Dissolution of ytterbium silicate into molten CMAS followed by precipitation of cyclosilicate, silicocarnotite, and Yb2Si2O7 at 1300 °C and Yb2Si2O7 at 1400 °C enabled CMAS to effectively infiltrate top coats, rendering the predominantly Yb2Si2O7 coating ineffective at arresting molten CMAS degradation.
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 17: Focus Issue: Sand-phobic Thermal/Environmental Barrier Coatings for Gas Turbine Engines , 14 September 2020 , pp. 2346 - 2357
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 12
- Cited by


